Magnesium orotate in the practice of a therapist and cardiologist
Magnesium enters the body with food. The main sources of its intake into the body are legumes and cereals, spinach, salads (including arugula), broccoli, and rhubarb. There is a lot of magnesium in pumpkin seeds and sesame seeds. Almonds, pine nuts and peanuts, cocoa and chocolate are especially rich in magnesium, but a significant increase in the consumption of these products can lead to weight gain due to their high calorie content. In addition, it must be remembered that no more than 30–40% of magnesium from food is absorbed. At the same time, for its good digestibility, the body also requires a sufficient amount of cofactors: lactic, aspartic, orotic acids and vitamin B6. In the development of nutritional magnesium deficiency, an important role is played by such factors as its low content in food, water, as well as excessive consumption of calcium, sodium, protein or fat from food, which significantly reduces the intake of magnesium in the body due to the formation of its non-absorbable complexes. The incidence of hypomagnesemia is quite high and ranges from 10 to 40%. To denote magnesium metabolic disorders, two terms are used: “magnesium deficiency,” which means a decrease in the total magnesium content in the body, and “hypomagnesemia,” which means a decrease in the concentration of magnesium in the serum (normally 0.8–1.2 mmol/l). Moderate magnesium deficiency in the body corresponds to its level in the blood serum of 0.5–0.7 mmol/l, severe (life-threatening) – below 0.5 mmol/l. There are primary (genetically determined) and secondary (nutritional, physiological, etc.) magnesium deficiency. The causes of magnesium deficiency can be various endocrine disorders (hypercalcemia, hyperaldosteronism, etc.), diabetes, chronic stress, alcoholism, as well as drug treatment, including long-term use of diuretics. Excretion of magnesium increases significantly with increasing levels of catecholamines and glucocorticosteroids. Also, significant losses of magnesium can occur with increased sweating [1, 4–6]. The main manifestations of magnesium deficiency in the body are presented in Table 1. The normal level of magnesium in the human body is recognized as a fundamental constant that controls human health. The study of intracellular molecular biokinetics revealed the presence of at least 290 genes and protein compounds in the human genome sequence that are capable of binding Mg2+ as a cofactor for many enzymes involved in more than 300 intracellular biochemical reactions. Magnesium ions stabilize substrate molecules - neutralize the negative charge of the substrate, the active center of the enzyme, contribute to the maintenance of the tertiary and quaternary structures of the enzyme protein molecule, facilitate the attachment of the substrate to the enzyme and thereby facilitate the chemical reaction, the magnesium - adenosine triphosphate (ATP) complex, stabilizing the molecule ATP promotes its attachment and “correct” orientation in the active center of the enzyme, weakening the phosphoester bond and facilitating the transfer of phosphate to glucose. In some cases, magnesium ion can help attach the coenzyme, promoting the activation of metalloenzymes. Magnesium contributes to the stability of the cell structure during growth and takes part in the process of regeneration of body cells. Mg2+ is a natural physiological antagonist of Ca2+, which causes it to have myotropic, antispasmodic and disaggregation effects, promotes the fixation of K+ in cells, ensuring the polarization of cell membranes, and controls the spontaneous electrical activity of nervous tissue and the conduction system of the heart. Magnesium affects the functional state of almost all organs and systems (Table 2) [4, 7–9]. The important role of magnesium in the development of endothelial dysfunction has been established. It has been shown that the administration of magnesium supplements can be effective after 6 months. significantly improve (almost 3.5 times more than placebo) endothelium-dependent dilatation of the brachial artery. At the same time, a direct linear correlation was also revealed - the dependence between the degree of endothelium-dependent vasodilation and the concentration of intracellular magnesium. One of the possible mechanisms explaining the beneficial effect of magnesium on endothelial function may be its antiatherogenic potential [4, 10]. Currently, magnesium preparations are widely used in the treatment of cardiovascular diseases. Experimental data indicate the important role of magnesium ions in the regulation of vascular tone and blood pressure. Magnesium has a hypotensive effect due to negative chrono- and inotropic effects, decreased vascular tone, inhibition of transmission in the autonomic ganglia, and inhibition of the vasomotor center. An inverse relationship has been established between the level of aldosterone and plasma renin, indicating that low magnesium levels are associated with increased activity of the renin-angiotensin-aldosterone system. Magnesium ions suppress the activity of the renin-angiotensin-aldosterone system, so severe vasoconstriction often occurs against the background of hypomagnesemia. On the contrary, with parenteral administration of magnesium, pronounced vasodilation is observed, comparable to the effect of calcium antagonists. Therefore, supplemental magnesium intake can be recommended for patients with arterial hypertension (HTN) who are at high risk of hypomagnesemia (for example, during therapy with thiazide diuretics) [4, 8, 14–19]. The use of magnesium-containing drugs for coronary heart disease (CHD) is also justified. The anti-ischemic effect of magnesium is due to the restoration of endothelium-dependent vasodilation, normalization of lipid spectrum indicators, improvement of the rheological properties of blood, a decrease in platelet aggregation activity, and a depressor effect on the inotropic function of the heart. According to epidemiological studies, magnesium deficiency in drinking water increases the risk of developing cardiovascular diseases (especially coronary heart disease) and sudden death. It is known that the myocardium of patients who died from cardiovascular pathology contains almost 2 times less magnesium than that of patients who died from other causes. Magnesium deficiency is associated with increased levels of atherogenic lipids. According to the ARIC study (The Atherosclerosis Risk in Communities), the incidence of coronary artery disease is higher in those individuals who have lower levels of magnesium in the blood. In Finland, as a result of the implementation of a government program to prevent magnesium deficiency in the country's population over the past 15 years, it has been possible to reduce the incidence of myocardial infarction (MI) in the population by almost 2 times. An analysis of pooled data from 7 randomized trials in 1301 patients with acute MI revealed a beneficial effect of magnesium on hospital mortality. The multicenter LIMIT-II study (2316 patients) revealed a reduction in the risk of death by 24% and a reduction in the risk of heart failure by 25% (in the group of patients with acute MI who received magnesium sulfate infusions in addition to standard therapy during the first 28 days). Magnesium deficiency was discovered in heart failure that developed against the background of hypertension and coronary artery disease. Therefore, additional magnesium intake as part of complex therapy can be recommended for patients with coronary artery disease and chronic heart failure [4, 8, 20–27]. In addition, it has been shown that against the background of magnesium deficiency, rhythm and conduction disturbances develop much more often during therapy with cardiac glycosides. Magnesium preparations are widely used in the treatment of arrhythmias due to digitalis intoxication due to their ability to restore the function of the potassium-sodium pump. According to the FHS study (Framinghem Heart Study), extrasystoles were detected in 5.5% of patients (n=3327, average age 44 years). At the same time, long-term hypomagnesemia correlates with a high incidence of ventricular extrasystoles, tachycardia, and ventricular fibrillation (p = 0.01). This pattern remained significant even after taking into account corrections for the mass of the left ventricle, including in volunteers without clinically significant disease. The PROMISE Study revealed a higher incidence of ventricular extrasystole and high mortality in the group of patients with hypomagnesemia compared to groups with normo- and hypermagnesemia [28–30]. Magnesium preparations have long been used as antiarrhythmic agents, combining the properties of antiarrhythmics I (membrane stabilizing) and IV (calcium antagonists) classes. Magnesium has a membrane-stabilizing effect and a depressor effect on the excitability and conductivity of the cell. Depletion of magnesium stores causes pronounced adverse effects on the myocardium. Violation of the content of potassium and magnesium ions and their ratio is a significant risk factor for the development of arrhythmias. Magnesium prevents the loss of potassium by the cell and reduces the variability of the QT interval, which is an unfavorable prognostic factor for the development of fatal arrhythmias. In addition, magnesium is able to inhibit sympathetic effects on the heart. As an antiarrhythmic, magnesium salts are most effective (drug of choice) for torsades de pointes due to their ability to inhibit the development of trace depolarizations and shorten the duration of the QT interval. Magnesium is also used both for congenital long QT syndrome and for its prolongation due to the use of class I antiarrhythmics. The results of the randomized multicenter placebo-controlled double-blind study MAGICA allowed us to consider magnesium and potassium preparations as the generally accepted European standard for the treatment of arrhythmias in patients taking cardiac glycosides, diuretics, and antiarrhythmics. The antiarrhythmic effect of magnesium drugs appears after 3 weeks. from the start of treatment and allows to reduce the number of ventricular extrasystoles by 12% and the total number of extrasystoles by 60–70% [4, 6, 8, 28, 31–35]. The use of magnesium preparations is effective for mitral valve prolapse. Thus, in patients who regularly took magnesium orotate, a significant change in echocardiographic parameters was established, indicating a positive effect of magnesium on dysplastic changes: a decrease in the depth of mitral valve prolapse, the degree of mitral regurgitation, the size of the left atrium and the frequency of myxomatous degeneration of the mitral valve leaflets [11, 36, 37]. Today, several drugs containing magnesium are used in clinical practice. One of the most successful is the drug Magnerot (pharmaceutical, Germany) - magnesium salt of orotic acid. One tablet contains 500 mg magnesium orotate (32.8 mg magnesium). To date, significant clinical material has been accumulated on the effectiveness of this drug in various fields of medicine, and primarily in cardiology and neurology. Orotic acid stimulates ATP synthesis. Since 90% of intracellular magnesium is bound to ATP, the relative increase in intracellular ATP storage through orotic acid improves magnesium fixation in cells. The use of drugs based specifically on organic magnesium salts, characterized by higher bioavailability compared to inorganic salts, is considered promising in clinical practice. Magnesium orotate, unlike inorganic magnesium oxide or sulfate, is more effective in correcting magnesium deficiency, especially in patients with acute coronary syndrome and heart failure, accompanied by cardiac arrhythmias. The cardioprotective effect of orotic acid is mediated through the regulation of the enzyme N-acetylglucosamine transferase, inhibition of intracellular phosphodiesterase and modulation of the coenzyme PQQ with anti-inflammatory, antioxidant and neuroprotective effects [7, 9, 38, 39]. When serum magnesium levels are below 0.5 mmol/l, CNS disorders of varying severity occur, which require intensive replacement therapy with magnesium preparations, such as Magnerot for oral use up to 3–6 g/day [7]. Thus, the inclusion of magnesium preparations, including magnesium orotate (Magnerot), in the combination therapy of cardiovascular diseases contributes to a more effective reduction of metabolic disorders, normalization of glycemic and lipid profiles, blood rheological properties, which overall leads to a decrease in blood pressure, prevention atherosclerosis and cardiovascular complications. The drug Magnesium orotate Magnerot should be considered the drug of choice in the complex treatment and prevention of angina pectoris, myocardial infarction, chronic heart failure, cardiac arrhythmias caused by magnesium deficiency, spastic conditions, atherosclerosis, and dyslipidemia. Many years of experience in the clinical use of magnesium preparations indicate their good effectiveness and high safety profile in the prevention and treatment of patients with various cardiological, neurological pathologies, as well as other diseases caused by magnesium deficiency.
Literature 1. Dreosti E. Magnesium status and health // Nutr. Rev. 1995. Vol. 53. P. 23–27. 2. Gorodetsky V.V., Talibov O.B. Magnesium preparations in medical practice. Small encyclopedia of magnesium. M.: Medpraktika, 2008. 43 p. 3. Petroianu A., Barquete J., Plentz EG Acute effects of alcohol ingestion on the human serum concentrations of calcium and magnesium // J. Int. Med. Res. 1991. Sep.-Oct. Vol. 19(5). P. 410–413. 4. Nedogoda S.V. The role of magnesium preparations in the management of therapeutic patients // Attending physician. 2009. No.6. pp. 16–19. 5. Schimatchek HF, Rempis R. Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals // Magnes. Res. 2001. Vol. 14. P. 283–290. 6. Shkolnikova M.A., Chuprova S.N., Kalinin L.A. and others. Metabolism of magnesium and the therapeutic value of its preparations. A manual for doctors. M.: Medpraktika, 2002. 32 p. 7. Shilov A.M., Osiya A.O. Magnesium preparations (Magnerot) and cardiovascular diseases in the practice of a primary care doctor // Difficult Patient. 2013. No. 12. P.12–19. 8. Morozova T.E., Durnetsova O.S. Magnesium preparations in cardiological practice // Attending physician. 2014. No. 4. pp. 95–99. 9. Korovina N.A., Tvorogova T.M., Gavryusheva L.P. The use of magnesium preparations for cardiovascular diseases in children // Attending physician. 2006. No. 3. P.10–13. 10. Shechter M., Sharir M., Labrador M. J. et al. Oral magnesium therapy improves endothelial function in patients with coronary artery disease // Circulation. Nov. 2000. Vol. 102. P. 2353–2358. 11. Martynov A.I., Urlaeva I.V., Akatova E.V., Nikolin O.P. The significance of magnesium deficiency in cardiology // Consilium Medicum. 2014. No. 01. pp. 43–46. 12. Akarachkova E.S. Magnesium deficiency. Cases from the practice of a neurologist // RMJ. 2010. No. 26 (1628). 13. Shilov A.M., Avshalumov A.Sh., Sinitsina E.N. and others. Metabolic syndrome and “magnesium deficiency”: features of the course and treatment // Doctor. 2008. No. 9.S. 44–48. 14. Cappuccio FP, Markandu ND, Beynon GW et al. Lack of effect of oral magnesium on high blood pressure: a double blind study // BMJ. 1985. Vol. 291. P. 235–238. 15. Ekmekci OB, Donma O., Tunckale A. Angiotensin-converting enzyme and metals in untreated essential hypertension // Biol. Trace Elem. Res. 2003. Vol. 95(3). P. 203–210. 16. Geleijnse JM, Witteman JC, Bak AA et al. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension // BMJ. 1994. Vol. 309 (6952). P. 436–440. 17. Mizushima S., Cappuccio FP, Nichols R., Elliott P. Dietary magnesium intake and blood pressure – a qualitative overview of the observational studies // J. Hum. Hypertens. 1998. Vol. 12. P. 447–453. 18. Shechter M., Sharir M., Labrador M. J. et al. Oral magnesium therapy improves endothelial function in patients with coronary artery disease // Circulation. Nov. 2000.Vol.102. P. 2353–2358. 19. Wirell MP, Wester PO, Segmayer BJ Nutritional dose of magnesium in hypertensive patients on beta blockers lowers systolic blood pressure: a double-blind, cross-over study // J. Intern. Med. 1994. Vol. 236. P. 189–195. 20. Liao F., Folsom AR, Brancati FL Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study // Am. Heart J. 1998. Vol. 136(3). P. 480–490. 21. Shechter M. Does magnesium have a role in the treatment of patients with coronary artery disease? //Am. J. Cardiovasc. Drugs. 2003. Vol. 3 (4). P. 231–239. 22. Lazebnik L.B., Drozdova S.L. Correction of magnesium deficiency in cardiovascular pathology // Cardiology. 1997. No. 5. pp. 103–104. 23. Diaz R, Paolasso EC, Piegas LS et al. on behalf of the ECLA (Estudios Cardiologicos Latinoamerica) collaborative group. Metabolic modulation of acute myocardial infarction. The ECLA glucose-insulin-potassium pilot trial // Circulation. 1998. Vol. 98. P. 2227–2234. 24. Fath-Ordoubadi F., Beatt KJ Glucose-insulin-potassium therapy for the treatment of acute myocardial infarction. An overview of randomized placebo – controlled trials // Circulation. 1997. Vol. 96. P. 1152–1156. 25. Shechter M., Hod H., Chouraqui P. et al. Magnesium therapy in acute myocardial infarction when patients are not candidates for thrombolytic therapy // Am. J. Cardiol. 1995. Vol. 75. P. 321–323. 26. Teo KK, Yusuf S, Collins R et al. Effects of intravenous magnesium in suspected acute myocardial infarction. Overview of randomized trials // Brit. Med. J. 1991. Vol. 303. P. 1499–1503. 27. Woods KL, Fletcheer S, Foffe C, Haider Y. Intravenous magnesium sulphate in suspected acute myocardial infarction. Results of the second Leicester Intravenous Magnesium Intervention Trial (LIMIT – 2) // Lancet. 1992. Vol. 343. P. 816–819. 28. Zehender M., Meinertz T., Just H. Magnesium deficiency and magnesium substitution. Effect on ventricular cardiac arrhythmias of various etiology // Herz. 1997. Jun. 22 (Suppl. 1). P. 56–62. 29. Tsuji H, Venditti FJ Jr, Evans JC et al. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study) // Am. J. Cardiol. 1994. Vol. 74. P. 232–235. 30. Eichhorn EJ, Tandon PK, Dibianco R. et al. The Study Group Clinical and prognostic significance of serum magnesium concentration in patients with severe chronic congestive heart failure: The Promise Study // J. Am. Coll. Cardiol. 1993. Vol. 21(3). P. 634–640. 31. Davydova S., Yarovoy S. Magnesium preparations in the treatment and prevention of supraventricular tachyarrhythmias in urological patients // Doctor. 2011. No. 9. pp. 44–49. 32. Chakraborti S., Chakraborti T., Mandal M. et al. Protective role of magnesium in the cardiovascular diseases: A review // Mol. Cell. Biochem. 2002. Vol. 238. P. 163–179. 33. Sueta CA, Clarke SW, Dunlap SH Effect of acute magnesium administration on the frequency of ventricular arrhythmia in patients with heart failure // Circulation. 1994. Vol. 89. P. 660–666. 34. Shilov A.M. and others. The use of magnesium preparations for the prevention of cardiac arrhythmias in patients with acute myocardial infarction // Ros. cardiol. magazine 2002. No. 1. P. 16–19. 35. Hoshino K., Ogawa K., Hishitani T. et al. Successful uses of magnesium sulfate for torsades de pointes in children with long QT syndrome // Pediatr. Int. 2006. Vol. 48(2). P. 112–117. 36. Martynov A.I., Akatova E.V., Nikolin O.P. Clinical effectiveness of magnesium orotate in patients with rhythm disturbances and arterial hypertension with mitral valve prolapse // Cardiovasc. therapy and prevention. 2009. No. 8. pp. 8–12. 37. Martynov A.I., Akatova E.V. Fifteen years of experience with the use of magnesium preparations in patients with mitral valve prolapse // Cardiology. 2011. No. 6. P. 60–65. 38. Torshin I.Yu., Gromova O.A., Fedotova L.E. and others. Chemoinformational analysis of the orotic acid molecule indicates anti-inflammatory, neuroprotective and cardioprotective properties of the magnesium ligand // Farmateka. 2013. No. 13. pp. 95–103. 39. Jellinek H., Takacs E. Morphological aspects of the effects of orotic acid and magnesium // Arzneimittelforschung. 1995. Vol. 45 (8). P. 836–842. 40. Altura BM Basic biochemistry and physiology of magnesium; A brief reviem // Magnesium and Frace Elements. 1991. Vol. 10. P. 167–171.
The role of magnesium orotate in the treatment of arrhythmic syndrome against the background of connective tissue dysplasia
In connective tissue dysplasia, one of the most common pathological disorders of the cardiovascular system is arrhythmic syndrome, which, manifesting itself from 5–6 years of age and maximally progressing in adolescence, can lead to the development of life-threatening cardiac arrhythmias in young people [1 , 2]. The leading role among modifiable risk factors for the formation of clinically significant arrhythmias in connective tissue dysplasia (CTD) is assigned to the imbalance of the autonomic nervous system [3]. At the same time, according to the results of most studies devoted to the study of the role of sympathetic tone in the pathology of the cardiovascular system associated with DST, there has been an idea of the prognostically unfavorable significance of hypersympathicotonia, including as an independent risk factor for the development of fatal arrhythmias [3–5]. It should be noted that the concentration of catecholamines in the blood plasma only indirectly characterizes the activity of the sympathetic nervous system. The severity of the physiological effect of catecholamines is largely determined by the degree of their binding to adrenergic receptors localized on the membranes of cells of organs and tissues, as well as erythrocytes [6, 7]. These circumstances served as the basis for considering β-adrenoreactivity of the erythrocyte membrane (β-ARM) as a systemic indicator of the body’s adrenoreactivity [7, 8].
Meanwhile, correlations have been established between the magnesium content in saliva and the presence of life-threatening arrhythmias [9]. In addition, literature data indicate the effectiveness of magnesium orotate dihydrate in reducing the average and maximum heart rate, the number of episodes of tachycardia and the frequency of paroxysms of supraventricular tachycardia, as well as reducing the severity of autonomic dysfunction syndrome, including in patients with mitral valve prolapse (MVP) [10–13].
The purpose of this study was to study the effect of magnesium orotate dihydrate on the state of adrenoreactivity in patients with arrhythmic syndrome associated with DST.
Material and research methods
The study included 72 patients with rhythm disturbances due to DST (38 men and 32 women) aged 18 to 35 years. Criteria for inclusion in the study: patients with heart rhythm disturbances and signs of connective tissue dysplasia; patient age from 18 to 40 years; voluntary informed consent of the patient to participate in the study. Exclusion criteria: patients with increased pressure in the pulmonary circulation, a history of traumatic injuries to the chest, diseases of the cardiovascular system (cardiomyopathy, congenital and acquired heart defects, myocarditis, heart failure, etc.), diseases of the thyroid gland, blood diseases, electrolyte disorders, acute or exacerbation of chronic inflammatory diseases, alcoholism, drug addiction, use of drugs that may affect the results of the study, contraindications to the use of magnesium orotate dihydrate, pregnancy and lactation, patient reluctance to participate in the study.
All patients who took part in the study underwent: general clinical examination (complaints, anamnesis, complete physical examination), electrocardiography (ECG) according to standard methods (Schiller electrocardiograph), Holter ECG monitoring for 24 hours (MT-200 monitor from Schiller ( Switzerland)), assessment of the level of β-adrenoreactivity of the body by changing the osmoresistance of erythrocytes under the influence of β-blockers using a diagnostic kit of reagents (AGAT Med, Moscow) [6, 8].
Based on the results of electrocardiographic research methods, groups of patients were formed taking into account the location of the ectopic focus: group I - 23 patients (11 men and 12 women), median age - 23 years (P25–75% = 22 ± 32), with registered polymorphic ventricular extrasystoles ( n = 11), monomorphic paired ventricular extrasystoles (n = 7), polymorphic paired ventricular extrasystoles (n = 5); Group II - 20 patients (9 men and 11 women), median age - 27.5 years (P25–75% = 23.5 ± 33), with registered ventricular extrasystole in the form of a monomorphic single with a frequency of no more than 30 ectopic complexes for any hour of monitoring (n = 13) and monomorphic single with a frequency of more than 30 extrasystolic complexes for any hour of monitoring (n = 7); Group III - 29 patients (20 men and 9 women), median age 25 years (P25–75% = 21 ± 29), with monomorphic single supraventricular extrasystole (SVE) with a frequency of less than 100 complexes for any hour of monitoring (n = 3) , monomorphic frequent SVE (n = 12), polytopic single SVE (n = 6), monofocal paroxysmal supraventricular tachycardia (n = 5), unstable paroxysm of supraventricular tachycardia (n = 2), atrioventricular paroxysmal tachycardia (n = 1). The control group consisted of 30 healthy volunteers (20 men and 10 women), median age was 23 years (P25–75% = 21 ± 29).
Patients of the study groups (I–III), after the examination, took magnesium orotate dihydrate for 4 months according to the instructions for use of the drug (2 tablets 3 times a day for 7 days, then 1 tablet 3 times a day daily until 4 months). After taking magnesium orotate dihydrate, the level of adrenoreactivity in the study groups was again assessed in order to study the restoration of the sensitivity of β-adrenergic receptors of erythrocytes.
Analysis for homogeneity of groups by age and gender was carried out using the nonparametric Mann–Whitney U Test for numerical data (age) and the χ2 test, taking into account the Yates correction, for dichotomous data (gender). The compared groups were comparable by gender and age.
For data processing, the Statistica 7.0 statistical software package and standard Microsoft Excel mathematical tables were used. In all statistical analysis procedures, the critical significance level p was taken equal to 0.05.
To determine the type of distribution of the studied samples, a graphical method was used to construct histograms with a visual assessment of the resulting distribution and using the Shapiro–Wilk W test. When significant criterion indicators were obtained, the null hypothesis about the correspondence of the analyzed data to the law of normal distribution was rejected. If the data did not correspond to the law of normal distribution, the median (Me) was used to describe the data instead of the arithmetic mean, indicating the interquartile range (the interval between the 25th and 75th percentiles). To identify differences between the analyzed groups, the Kruskal–Wallis ANOVA method was used; to clarify the identified differences, the method of pairwise comparison of groups was used using the nonparametric Mann–Whitney U Test. Taking into account the multiple comparisons, a Bonferroni correction was applied to clarify the level of significance at which p values below the calculated ones were regarded as significant.
Results and discussion
In order to restore the sensitivity of β-adrenergic receptors in patients with arrhythmic syndrome associated with DST, magnesium orotate dihydrate was chosen, which has a number of advantages: magnesium ions participate in the processes of cell excitation, being a physiological calcium antagonist; with a deficiency of magnesium ions, the exchange of cations on the cell membrane is disrupted, often leading to electrical overexcitability; Magnesium ions are necessary for the normal metabolism of neurotransmitters (catecholamines, tyrosine, dopamine, norepinephrine, serotonin, γ-aminobutyric acid). In addition, a number of studies have proven a deficiency of this macroelement in patients with CTD [12, 13].
In general, patients noted good tolerability of the drug; no cases of adverse events were recorded. At the end of the course of therapy, patients in the study groups showed significant positive dynamics in a number of subjective manifestations. Thus, the frequency of registration of asthenic complaints decreased (p < 0.01), less than a third of patients complained of palpitations, interruptions in heart function became less frequent and were noted by less than half of the patients, cardialgia bothered only every fourth patient, and the tolerability of moderate physical activity (p < 0.001).
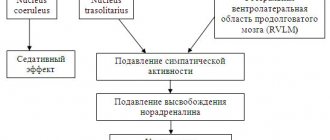
According to 24-hour ECG monitoring, a decrease in the number of ventricular extrasystoles in groups I and II was noted in a third of patients, in every seventh patient from group I, high-grade extrasystoles disappeared, in a quarter of patients from group II, ventricular extrasystoles were not registered after treatment (p < 0.05 ). In group III, 10 patients, instead of polytopic and paired supraventricular extrasystoles, had monotopic and single ectopies. In our opinion, the antiarrhythmic activity of magnesium orotate in this case was associated primarily with the fact that magnesium, being a natural calcium antagonist, has a membrane-stabilizing property inherent in class I antiarrhythmic drugs, thereby helping to slow down the loss of potassium by the cell, reducing the dispersion of the QT interval length by ECG, as well as inhibition of the sympathetic effect on the heart [12, 14].
During the study of β-adrenoreception of erythrocyte membranes by the method of assessing changes in osmoresistance of erythrocytes under the influence of β-adrenergic blockers in patients of the study groups, it was revealed that the median indicator of β-adrenoreactivity of erythrocyte membranes (β-ARM) in group I was 52.07 conventional units (P25– 75% = 48.03 ± 55.22), in patients of group II 43.54 conventional units (P25–75% = 39.56 ± 45.75), in group III 36.7 conventional units (P25–75% = 35.1 ± 40.64), while in the group of healthy volunteers it was significantly lower - 17.43 conventional units (P25–75% = 13.22 ± 22.08), without going beyond normal values (2–20 conventional units). When conducting pairwise comparisons between groups, the following results were obtained. The highest values of the β-ARM indicator were recorded in patients with ventricular heart rhythm disturbances, with maximum values in the group of patients with a high gradation of extrasystoles according to Lown, which significantly distinguished them from the group with supraventricular rhythm disturbances and the control group (Table).
Thus, in patients with DST, higher values of β-ARM of erythrocyte membranes were revealed than in the group of healthy volunteers, which corresponded to the desensitization of adrenergic receptors (p < 0.001).
After a course of magnesium orotate dihydrate for 4 months, a decrease in β-ARM was noted in patients with arrhythmic syndrome against the background of DST (groups I–III), which could indicate a process of adrenergic receptor resensitization. Thus, in group I, the median β-ARM indicator was 40.42 conventional units (P25–75% = 35.1 ± 45.7), in group II - 32.73 conventional units (P25–75% = 28.83 ± 36.62), in group III - 28.05 conventional units (P25–75% = 24.3 ± 31.09). In general, in research groups I and II, the target levels of β-ARM, at which cells are sensitive to β-blockers, were achieved in more than half of the patients; however, in every 10th patient, despite a decrease in individual ARM values, further correction was necessary desensitization of adrenergic receptors. At the end of the course of therapy in group III, not a single case was registered with β-ARM values higher than 40 conventional units, while in a third of patients this indicator was within 30–40 conventional units. When comparing the β-ARM indicator in all study groups (I–III) before and after the end of the course of treatment, statistically significant positive dynamics were noted (Wilcoxon Test, p = 0.00000), however, normal values (according to the conditions of the method) were not achieved, which speaks of the need to further search for ways of early pharmacological correction of the process of adrenergic receptor desensitization.
Thus, in patients with DST, higher values of the β-adrenoreception index of erythrocyte membranes are detected than in the group of healthy volunteers, which corresponds to the desensitization of adrenoreceptors. The causes of desentification in most cases are not precisely established. A number of studies provide evidence that the loss of sensitivity of adrenergic receptors is influenced by stress [15, 17]. Moreover, the rate of desensitization and resentization of adrenergic receptors is largely comparable to the duration of exposure to the stressor [17]. In addition, in patients with DST, according to some researchers, the processes of lipid peroxidation are increased [5, 18, 19], leading to a more rapid process of desensitization of adrenergic receptors in these patients. Meanwhile, according to the results of the study, the use of magnesium orotate dihydrate in patients with arrhythmic syndrome against the background of DST is pathogenetically justified and effective. The administration of magnesium orotate dihydrate led to an improvement in subjective well-being and electrophysiological parameters of cardiac activity (reduced the severity and frequency of registration of arrhythmic episodes) in the majority of patients; increased the osmoresistance of erythrocytes in the presence of β-blockers, which may indicate the process of resentization of adrenergic receptors, which is very important in the management of patients with arrhythmic syndrome.
conclusions
- In patients with arrhythmic syndrome against the background of DST, there is desensitization of adrenergic receptors with maximum values of the indicator in the group of patients with high-grade ventricular extrasystoles (p < 0.001).
- Taking magnesium orotate dihydrate for 4 months restores the sensitivity of adrenergic receptors (Wilcoxon Test, p = 0.0000) in patients with cardiac arrhythmias due to DST.
- It is advisable to include a method for assessing the body’s adrenoreactivity based on β-ARM value in the examination program for patients with cardiac arrhythmias due to DST, which will allow individualizing the selection and assessment of the effectiveness of antiarrhythmic therapy in this category of patients.
Literature
- Clinical recommendations of the Russian scientific medical society of therapists for the diagnosis, treatment and rehabilitation of patients with connective tissue dysplasia (first revision) // Medical Bulletin of the North Caucasus. 2018; 1.2 (13): 137–210. https://doi.org/10.14300/mnnc.2018.13037.
- Shilova M. A., Mamedov M. N. Sudden cardiac death in young people: risk factors, causes, morphological equivalents // Cardiology. 2015; 7 (55): 78–83. https://dx.doi.org/10.18565/cardio.2015.7.78–83.
- Druk I.V., Nechaeva G.I., Oseeva O.V. et al. Personalized assessment of the risk of developing adverse cardiovascular complications in young patients with connective tissue dysplasia // Cardiology. 2015; 3: 75–84. 10.18565/cardio.2015.3.75–84.
- Zemtsovsky E. V., Tikhonenko V. M., Reeva S. V. Functional diagnostics of the state of the autonomic nervous system. St. Petersburg: INKART, 2004.
- Nechaeva G.I., Yakovlev V.M., Druk I.V. Tikhonova O.V. Heart rhythm disturbances in undifferentiated connective tissue dysplasia // Attending Physician. 2008; 6:43–47.
- Sergeev P. V., Shimanovsky N. L. Receptors of physiologically active substances. M., 1987. 400 p.
- Bristow MR β-Adrenergic receptor blockade in chronic heart failure // Circulation. 2000; vol. 10:558–569.
- Malkova M.I., Bulashova O.V., Khazova E.V. Determination of the body’s adrenoreactivity by adrenoreception of the cell membrane in cardiovascular pathology // Practical Medicine. 2013; 3 (71): 20–23.
- Tikhonova O. V., Drokina O. V., Moiseeva N. E., Nechaeva G. I., Martynov A. I. Assessment of the information content of methods for determining magnesium content in the body using the example of patients with signs of connective tissue dysplasia // Archives of Internal Medicine . 2014; 1 (15): 19–24.
- Martynov A.I., Akatova E.V., Nikolin O.P., Urlaeva I.V. Effect of magnesium orotate on cardiovascular risks // Therapy. 2016; 5:52–57.
- Martynov A.I., Akatova E.V. Fifteen years of experience with the use of magnesium preparations in patients with mitral valve prolapse // Cardiology. 2011; 6:60–65.
- Torshin IY, Gromova OA, Kalacheva AG, Oshchepkova EV, Martynov AI Meta-analysis of clinical trials of cardiovascular effects of magnesium orotate // Ter. Archive. 2015; 87(6):88–97. 10.17116/terarkh201587688–97.
- Sologova S. S., Maksimov M. L., Tarasov V. V. Pharmacological effectiveness of magnesium preparations. Magnesium orotate (Magnerot) in clinical practice // RMJ. Medical Review. 2014; 127: 1966.
- Gorodetsky V.V., Talibov O.B. Magnesium preparations in medical practice. Small encyclopedia of magnesium. M.: Medpraktika, 2003. 44 p.
- Torda T., Yamaguchi I., Kopin IJ, Axelrod J Quinacrine-blocked desensitization of adrenoreceptors after immobilization stress or repeated injection of isoproterenol in rats // J. Pharmacol. Exp. Ther. 1981; vol. 216:334–338
- Zemtsovsky E.V. Sports medicine. St. Petersburg: Hippocrates, 1995. 448 p.
- Stryuk R.I., Dlusskaya I.G. Adrenoreactivity and the cardiovascular system. M.: Medicine, 2003. 160 p.
- Ponomareva D. A., Nagaeva T. A., Balasheva I. I. Features of the state of the digestive tract and lipid peroxidation system in connective tissue dysplasia in schoolchildren according to the results of screening examinations // Siberian Bulletin of Hepatology and Gastroenterology. 2007; 21: 71–73.
- Nechaeva G.I., Martynov A.I. Connective tissue dysplasia: cardiovascular changes, modern approaches to diagnosis and treatment. M: Medical Information Agency LLC. 2017; 400.
E. N. Loginova1, Candidate of Medical Sciences Yu. V. Moskvina, Candidate of Medical Sciences G. I. Nechaeva, Doctor of Medical Sciences, Professor I. V. Druk, Doctor of Medical Sciences, Professor A. A. Semenkin, Doctor of Medical Sciences, Professor M. I. Shupina, Candidate of Medical Sciences Yu. V. Tereshchenko, Candidate of Medical Sciences
Federal State Budgetary Educational Institution of Higher Education Omsk State Medical University of the Ministry of Health of the Russian Federation, Omsk
1 Contact information
The role of magnesium orotate in the treatment of arrhythmic syndrome against the background of connective tissue dysplasia / E. N. Loginova, Yu. V. Moskvina, G. I. Nechaeva, I. V. Druk, A. A. Semenkin, M. I. Shupina, Yu V. Tereshchenko For citation: Attending physician No. 12/2018; Page numbers in the issue: 50-53 Tags: cardiovascular system, arrhythmic syndrome, young patients
It has been known for more than a century that the metabolism of macronutrients (such as fats, proteins and carbohydrates) is greatly hampered by a deficiency of micronutrients (metal ions, organoelement compounds, vitamins) in the body. Micronutrients directly affect physiological processes, because in the vast majority of cases, they are cofactors of enzymes or factors stabilizing the spatial structures of proteins and RNA. The functioning of the heart and the entire cardiovascular system is almost impossible without a number of certain micronutrients, in particular magnesium.
In the case of magnesium, large-scale evidence-based studies have been conducted. For example, the correlation between mortality from cardiovascular diseases and magnesium levels in drinking water has long been known. A relatively recent Swedish study of 1679 patients found that the risk of death from myocardial infarction was lower in the group with high plasma magnesium levels (>0.83 mmol/L) than in the group with lower levels (<0.75 mmol/l). The risk of death from acute myocardial infarction in relation to magnesium levels in drinking water was 0.64 (95% confidence interval [CI] 0.42–0.97) for the group with the highest levels.
An 8-year study of a cohort of 5511 participants 28–75 years of age without hypertension at baseline found that the risk of hypertension (systolic blood pressure [SBP] ≥140 mmHg or diastolic blood pressure [DBP] ≥ 90 mm Hg) decreased with increasing magnesium levels in daily urine. Higher levels of magnesium in urine indicate higher intake from food and medications. Every 2 mmol increase in magnesium levels was associated with a 21% reduction in the risk of hypertension (odds ratio [OR], 0.79; 95% CI, 0.71 to 0.88).
A meta-analysis of prospective cohort studies included a total of 532,979 participants from 19 studies, incl. 19,926 cases of established cardiovascular pathology. Comparison of subgroups with the highest and lowest levels of dietary magnesium intake showed that in participants in subgroup 1, the risk of cardiovascular diseases decreased by an average of 15% (OR - 0.85; 95% CI - 0.78–0. 92; Fig. 1). Grouping patients according to plasma magnesium levels indicated a 23% reduction in risk at high magnesium concentrations (OR, 0.77; 95% CI, 0.66–0.87). The confirmed cardioprotective effect of magnesium was dose-dependent - an increase in the level of magnesium in the blood plasma by 0.2 mmol/l corresponded to a decrease in the risk of cardiovascular pathology by an average of 25% (Fig. 1).
Thus, from the point of view of the results of clinical studies, etc. evidence-based medicine, the cardioprotective effect of magnesium is beyond doubt. From the point of view of the physiology of systems, with a lack of magnesium, the heart muscle becomes unable to withstand significant physical stress and wears out faster, which leads to a heart attack due to physical overload of the myocardium. Magnesium supports energy and plastic processes, stabilizes ATP levels, participates in the oxidation of fatty acids, glycolysis and protein biosynthesis, the synthesis of nitric oxide in the vascular endothelium, etc. Magnesium is also a physiological regulator of cell excitability and is absolutely necessary for the normal functioning of depolarization processes of nerve and muscle cells .
Magnesium-dependent proteins that support cardiac muscle function
It is known that magnesium levels significantly affect myocardial contractility and a deficiency of this mineral leads to weakened cardiac function and arrhythmias. For example, a study of the effects of long-term magnesium fasting in rats (8 weeks on a magnesium-depleted diet) showed a decrease in intracellular adenine nucleotides, increased levels of creatine phosphate, and a decrease in the strength of myocardial contractions without a change in heart rate. These and other consequences of magnesium deficiency are mediated by a number of magnesium-dependent proteins that directly affect the function of the heart muscle. These proteins can be subdivided into enzymes that regulate the levels of signaling molecules, magnesium-dependent proteins that control the flux of cations across membranes, and proteins that support the cytoskeleton of muscle cells (Fig. 2).
In total, at least 20 proteins are involved in magnesium-dependent regulation of connective tissue. Possible mechanisms of the effect of magnesium deficiency on the synthesis and degradation of connective tissue include activation of matrix metalloproteinases, lysyl oxidase, glutaminase, inhibition of the synthesis of collagen, elastin and hyaluronan, as well as removal of magnesium inhibition of metalloproteinases and hyaluronidases that degrade connective tissue.
With Mg2+ deficiency, protein synthesis of connective tissue slows down, the activity of metalloproteinases increases and the extracellular matrix progressively degrades, because the structural support of the tissue (in particular, collagen fibers) is destroyed faster than it is synthesized. The structure of connective tissue (in particular, cartilage) can also be influenced by magnesium-dependent proteins of cell proliferation signaling pathways and, above all, activin receptor type 2B (ACVR2B), which activates SMAD transcriptional regulators, which leads to activation of fibroblasts and acceleration of wound healing.
“Energy metabolism” is a fairly broad concept, including anabolic and catabolic processes from proteins, fats and carbohydrates, ultimately leading to the accumulation of a reserve of cellular ATP, this universal energy transfer molecule in biological systems. Only 1 mM of magnesium ions is in the body in a free state, the rest of the biometal is associated with proteins and soluble compounds, such as ATP, skeletal muscle myosin, myocardial troponin-C, amino acids (in particular, glycine, alanine, aspartic acid, etc.) .d.) and various enzymes. The interactions of magnesium with ATP are most important for energy metabolism: Mg2+ stabilizes the ATP molecule by neutralizing the excess negative charge of phosphates.
In addition to affecting the interaction of magnesium with ATP, which is important for energy metabolism, magnesium deficiency also negatively affects the functioning of many cardiovascular proteins that support energy metabolism and require magnesium as a cofactor.
These magnesium-dependent proteins are involved in the synthesis of important coenzymes, in the metabolism of carbohydrates (in particular, in glycolysis), and in mitochondria they are involved in the metabolism of pyruvate and fatty acids. A decrease in the activity of these enzymes (primarily glycolytic) is the most likely explanation for the formation of insulin resistance.
On the therapeutic use of organic magnesium salts
Magnesium has a wide range of effects on metabolism, structure and function of the heart and the entire cardiovascular system. Taking into account that the incidence of magnesium deficiency in various populations is 30–50%, it becomes clear that a significant number of cardiac patients have it to one degree or another. Accordingly, many of the molecular functions described above will be slowed, resulting in greater susceptibility to adverse cardiovascular events. Restoring the functional state of the heart muscle will also be difficult with magnesium deficiency (due to an imbalance of apoptosis and proliferation, weakened connective tissue structure, etc.).
In addition to the enduring role of magnesium in the physiology of the cardiovascular system, there is another aspect of the issue regarding the pharmacological correction of magnesium deficiency - the bioavailability of magnesium in various drugs. Natural sources of magnesium – vegetables, fruits, nuts, etc. – have the best bioavailability. However, replenishment of its deficiency in diseases of the cardiovascular system cannot be carried out only through diet correction. Pharmacological support with magnesium drugs is extremely important. Inorganic magnesium preparations, such as magnesium sulfate, are characterized by extremely low bioavailability and a number of pronounced side effects. The use of second-generation drugs based on organic magnesium salts and having high bioavailability in magnesium pharmacotherapy is much more promising. One such drug is magnesium orotate, a salt of magnesium and orotic acid. Orotic acid is one of the products of pyrimidine biosynthesis.
In pharmacology, salts of orotic acid are used as a carrier of minerals, because orotic acid increases the cellular bioavailability of cations and has a number of additional advantages over inorganic salts. For example, magnesium orotate does not react with stomach acid and does not have a laxative effect. The effectiveness of magnesium orotate has been shown in conditions accompanied by magnesium deficiency, incl. in patients undergoing coronary surgery. It has antiarrhythmic, vasodilating and cardioprotective effects. By increasing the resistance of myocytes to ischemia, magnesium orotate has a beneficial effect on the clinical course of myocardial infarction and heart failure.
Experiments show that orotic acid and magnesium orotate help improve the lipid profile of blood plasma (the ratio of low-density lipoproteins to high-density lipoproteins). The effect of orotic acid on lipids is most likely also mediated by purinergic receptors, which act on the transcription of many genes through phosphorylation of the transcriptional regulator CREB.
The results of clinical use of magnesium orotate confirm the results of basic research. A study of 79 patients with severe heart failure treated with magnesium orotate found that their one-year survival rate was 76%, compared with 52% in the placebo group. The use of magnesium orotate increases exercise tolerance in patients with coronary artery disease (CHD). Taking the drug by elderly patients with coronary artery disease improved parameters of quality of life and psycho-emotional status. The positive effect of magnesium orotate in these cases may be associated with vasodilation and increased energy metabolism of cardiomyocytes. From a physiological point of view, the combined use of magnesium and orotate is synergistic. In practice, it has been shown that the use of magnesium orotate in complex therapy has a positive effect on the survival and quality of life of patients with coronary artery disease.
In the case of idiopathic mitral valve prolapse (MVP), which is often considered a type of connective tissue dysplasia, the positive effect of magnesium orotate is provided not only through vasodilation and improvement of energy metabolism, but also due to structural changes in connective tissue. Systematic use of magnesium orotate has proven effective in the treatment of patients with connective tissue dysplasia of the heart syndrome (in particular, MVP and abnormal chordae tendineae). A study of 144 patients with idiopathic MVP showed that the use of magnesium orotate led to a decrease in maximum SBP and DBP, as well as mean DBP, and a decrease in the number of episodes of tachycardia. Six-month therapy with magnesium orotate completely or partially relieves symptoms of MVP in more than half of patients.
Magnesium citrate is one of the organic salts used to make modern magnesium-containing preparations. Since citrate is an organic and highly soluble form of magnesium, this is largely responsible for its high bioavailability. However, good solubility in water is not the only feature of magnesium citrate, which is also characterized by a number of specific molecular effects. This is the participation of magnesium as a central substrate of the Krebs cycle (which even has an alternative name - the “citrate cycle”), interaction with proteins that transport dicarboxylates, and finally the effects caused by the physicochemical characteristics of the citrate molecule itself. It should be emphasized that all citrate metabolites are essential endogenous molecules. The almost complete utilization of citrate (conversion into carbon dioxide and water) makes it an ideal carrier of magnesium.
An analysis of the literature on the medical use of magnesium citrate preparations shows that it has been used in therapy for more than 50 years and is used to prevent the formation of kidney stones (25 studies), in the treatment and prevention of hypomagnesemia and hypokalemia (8), in vascular diseases (5) and in obstetrics (4 studies). Other medical indications for the use of magnesium citrate (3 studies) include normalization of bone mineral density, treatment of restless legs syndrome and bronchial asthma.
The mechanisms of molecular functions of magnesium-dependent proteins in cardiac tissue include, in particular, control of ion channels, regulation of levels of signaling molecules, glycolysis, vesicular transport, intracellular signaling from cytokines, influence on connective tissue metabolism, apoptosis and cell division. The formulated generalized picture of the effect of magnesium on the cardiovascular system at the molecular level indicates multiple mechanisms through which the therapeutic effect of organic magnesium preparations is carried out in cardiac patients. Replenishing magnesium deficiency through nutritional supplements or a properly balanced diet will help restore the normal functioning of these molecular cascades, thus having a positive effect on the cardiovascular system. In general, magnesium has a positive effect on energy metabolism, connective tissue structure and vascular tone, helping to reduce the level of catecholamines in plasma.