Davydov M.I., Polotsky B.E., Machaladze Z.O., Tyulyandin S.A., Karseladze A.I., Savelov N.A., Akhmedov B.B. (Russian Scientific Research Center named after N.N. Blokhin)
In the materials of the Russian Scientific Research Center named after. N.N.Blokhina 1980-2005 reflects the experience of diagnostics, surgical and combined treatment of over 500 patients. Among the analyzed cases were tumors of organs and tissues of the mediastinum itself: thymus (16.9%), neurogenic (17.7%) and mesenchymal (10.4%) tumors; neoplasms from tissues dystopic into the mediastinum (extragonadal germ cell tumors, teratomas, dermoid cysts) - 22.7%, as well as processes with isolated damage to the mediastinal lymph nodes: Hodgkin's lymphoma and non-Hodgkin's lymphoma (25.7%), cases of Beck's sarcoidosis ( 29), angiofollicular hyperplasia (4), etc.
Generalization of the experience accumulated, especially in the 1990s - 2000s, taking into account modern capabilities of clinical oncology, made it possible to optimize clarifying diagnostics and justify the expansion of indications for surgical and combined treatment, repeated and palliative operations. The results of operations for mediastinal tumors, primarily with massive tumor lesions associated with compression of the respiratory tract, esophagus, and great vessels (subject to proper resuscitation and anesthesia support) are characterized by relatively low mortality, improved quality of life and increased life expectancy.
GENERAL PROVISIONS (CLINICAL, DIAGNOSIS, TREATMENT...)
The materials of the Russian Cancer Research Center confirm the known information about the localization and predominantly malignant nature of mediastinal tumors. The ratio of malignant and benign neoplasms was 3:1. Thymomas, lymphomas, extragonadal germ cell tumors (EGT), mesenchymomas were localized in the anterior mediastinum, neurogenic tumors - in the posterior; a discrepancy between the localization and structure of the tumor was noted in 15% of patients with neurogenic tumors - in cases where the tumor was localized in the anterior mediastinum. Determining the initial localization of the process was difficult in “giant” tumors, when (in 10-20% of cases) lesions of both the anterior and posterior mediastinum were observed with the process spreading to most of the hemithorax.
The duration of clinical manifestations ranged from several months to 3 years. For small tumors not associated with adjacent anatomical structures, no clinical manifestations were observed (up to 30-40% of cases). In the remaining observations, manifestations caused by the presence of a tumor were recorded - myasthenia gravis (5%), hoarseness (often associated with VHO), mediastinal compression syndrome (up to 30-40% of cases, especially in malignant processes); as well as various nonspecific symptoms (fever, cough, pain and a feeling of compression behind the sternum, etc.). X-ray examination, of course, made it possible to determine the location and size of the tumor. CT and MRI were used to clarify the topography of neoplasms and identify connections with adjacent anatomical structures. Sensitivity. the specificity and accuracy * of these methods corresponded to 90-80%, reaching 97-98% of the significance of the assessment - such as MRI in diagnosing the prevalence of thymomas. Increased levels of serum tumor markers (AFP and hCG) were observed in nonseminoma germ cell tumors of the mediastinum. Morphological confirmation of the diagnosis, in addition to puncture biopsy (informativeness for lymphoproliferative diseases corresponds to 48%), was achieved by surgical diagnostic methods. At the same time, instead of mediastinoscopy and parasternal mediastinotomy (16 and 43 studies in the 1990s) in the 2000s. Videothoracoscopy began to be used (53 studies).
It should be noted that modern diagnostic capabilities make it possible to establish a morphological diagnosis in all cases of lymphoproliferative diseases involving the mediastinum. At the same time, the aggressiveness of the treatment methods used forces us to consider it unacceptable to attempt treatment for these neoplasms without a morphological diagnosis.
The surgical method is the basis of treatment for patients with mediastinal tumors.
Exceptions include malignant extragonadal germ cell and lymphoproliferative diseases, the treatment of which is based on conservative treatment methods. Considering the low effectiveness of chemo-radiation therapy for other mediastinal neoplasms, surgical treatment, including for palliative purposes, with repeated operations, contributes to a significant prolongation of life. Performing a lateral thoracotomy in 73% of cases provided the possibility of tumor removal. The localization of the process, of course, determined the characteristics of the surgical approach. Thus, for neurogenic tumors growing in an “hourglass” shape, thoracotomy was supplemented with a one-stage laminectomy for radical removal of the intrathoracic and intravertebral parts of the tumor - according to N. Guleke (1916) as modified by H. Grillo et al. (1983). To remove locally advanced and “giant” tumors, bilateral thoracotomy, complete longitudinal sternotomy (4), and other combined approaches were performed.
Introduction
“Mediastinal tumors” is a collective term used to designate neoplasms of various origins, originating from dissimilar tissues and united into one nosological form only due to common anatomical boundaries.
The mediastinum is the part of the thoracic cavity bounded on the sides by the mediastinal pleura, behind by the thoracic spine and the necks of the ribs, in front by the sternum, below by the diaphragm, and above by the level of the upper aperture of the chest.
From an anatomical point of view, the mediastinum is a single space, but for practical reasons it is usually divided into sections. The conventional lines proposed by B. Felson [1] follow landmarks on lateral X-ray films of the chest, dividing the mediastinum into three sections: anterior, middle and posterior. In subsequent years, the boundaries and conventional sections of the mediastinum were not subject to correction [2]. In the horizontal plane, we considered lines passing along the level of the upper border of the shadow of the aortic arch and the projection of the inferior pulmonary vein; in the vertical - along the anterior and posterior walls of the trachea, as well as the posterior contour of the heart shadow. Thus, the mediastinum could be conditionally divided into 9 sections: anterosuperior, anteromedial, anterior-inferior, middle-superior, middle, middle-inferior, posterosuperior, posteromedial, posteroinferior. In practice, the terms “anterior and posterior mediastinum” are more often used.
The introduction of computed tomography (CT) in the diagnosis of mediastinal pathology required the development of new guidelines in assessing the boundaries of this area. Thus, the Japanese Association for the Study of Thymic Pathology (JART) in 2009 proposed the boundaries of the mediastinum based on landmarks visualized on transverse planar sections of chest CT. There are 4 sections of the mediastinum: upper, anterior (prevascular space), middle (peritracheoesophageal zone) and posterior (paravertebral region) [3].
Superior mediastinum
is defined as a space limited from above by a conventional horizontal plane passing along the level of the chest aperture; from below - at the level of the projection of the intersection of the left brachiocephalic vein of the trachea along the midline of the chest (Fig. 1, 2).
Rice. 1. Upper mediastinum. Axial scan of a CT image in a soft tissue window at the level of the sternoclavicular joint. Rice. 1. Upper mediastinum. Axial scan of a CT image in a soft tissue window at the level of the sternoclavicular joint.
Rice.
2. Upper mediastinum. Axial scan of a CT image in a soft tissue window at the level of the left brachiocephalic vein. Rice. 2. Upper mediastinum. Axial scan of a CT image in a soft tissue window at the level of the left brachiocephalic vein. In front, this section of the mediastinum is limited by the sternum, and behind - by the vertebral bodies, their transverse processes and the heads of the ribs. To the anterior mediastinum
include the zone bounded above by the lower wall of the left brachiocephalic vein (i.e., the lower border of the upper mediastinum), below by the diaphragm, in front by the sternum, behind by the pericardium, anterior semicircles of the walls of the aortic arch, brachiocephalic trunk, superior vena cava, pulmonary trunk, and on the sides by the mediastinal pleura, outer semicircles of the walls of the internal mammary vessels (Fig. 3-5).
Rice. 4. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the tracheal bifurcation. Rice. 4. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the tracheal bifurcation.
Rice. 5. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the pulmonary arteries. Rice. 5. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the pulmonary arteries.
Rice.
3. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the aortic arch. Rice. 3. Anterior, middle and posterior mediastinum. Axial scan of a CT image in a soft tissue window at the level of the aortic arch. Middle mediastinum region
begins superiorly from the inferior border of the superior mediastinum, extending to the diaphragm (inferior border). The anterior border of the middle section of the mediastinum runs along the posterior semicircles of the walls of the great vessels of the mediastinum, and the lower border is 1 cm posterior to the anterior semicircle of the vertebral bodies and the descending aorta (see Fig. 3-5).
Posterior mediastinum
starts from the level of the lower border of the upper mediastinum to the diaphragm. Its anterior border is a conventional line drawn along the posterior border of the middle mediastinum (see above). As in the upper mediastinum, the posterior border runs along the bodies and transverse processes of the vertebrae, and the heads of the ribs (see Fig. 3-5).
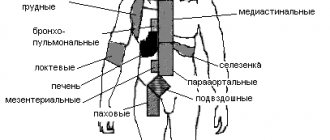
The following structures and organs are located in the mediastinum: the thymus, trachea, main bronchi, aorta, esophagus, heart and its large arterial and venous great vessels, vagus and phrenic nerves, thoracic lymphatic duct. Between these formations there is tissue of the mediastinum itself.
, which is loose fibrous connective and adipose tissue, small blood and lymphatic vessels, nerves, and clusters of lymph nodes (see Fig. 1-5).
The source of development of mediastinal tumors can be:
• organs located and passing through the mediastinum;
• tissues delimiting the mediastinum;
• tissues located between organs, i.e. the mediastinal tissue itself
;
• tissues displaced into the mediastinum due to impaired embryogenesis (the rudiments of the thyroid and parathyroid glands, undifferentiated germ cells).
In the mediastinum there are also pathological processes and developmental defects, accompanied by the formation of cysts, which should be taken into account when carrying out differential diagnosis in patients with identified formations of this localization.
FEATURES DUE TO THE LOCALIZATION OF NEW FORMS
Tumors of the thymus gland. Of 85 observations, benign neoplasms were diagnosed in 44.7% of cases, and malignant ones in 55.3%. The clinical course of the disease was not always determined by the histological structure of the tumor and the nature of its growth. 70 (82.4%) patients underwent surgery; the rest received conservative treatment due to the prevalence of the tumor process.
Radical operations accounted for 59 (84.3%) cases; in the remaining patients, tumor removal was performed for palliative purposes; in 6 (8.6%) the operation was limited to exploratory thoracotomy. The adequate scope of surgical intervention for tumors of the thymus gland is thymomectomy - removal of the tumor and all tissue of the thymus gland with fatty tissue and lymph nodes of the anterior mediastinum. This volume of surgery was performed in 44 patients. In addition, 15 patients underwent extended-combined thymectomy due to tumor invasion into surrounding structures. Additional radiation and chemotherapy were carried out in 19.2% of cases (mainly for stages III-IV thymomas of types B2, B3 and C). Long-term results of surgical treatment. 84.8% of patients lived more than 3 years after radical surgical treatment, 82.6% lived more than 5 years, and 73.9% of patients lived more than 10 years. Long-term results were determined by the extent of the process (capsular invasion, pleural damage), and the histological type of tumor. Thus, thymomas of types A and B1 have the most favorable prognosis (90% of patients survive the 10-year period). For thymomas of types AB, B2 and B3, the 5-year survival rate was 64-68%; the prognosis for type C thymomas is significantly worse – 32%. In the absence of invasive growth, the histological form of thymoma does not affect the long-term results of surgical treatment. Thymomas with invasion within the capsule are clinically malignant and require postoperative radiation treatment.
Neurogenic tumors (89 cases) are divided into two histogenetic groups: neoplasms from nerve tissue cells (22; 24.7%) and from peripheral nerve sheaths (67; 75.3%). Benign tumors (schwannomas, neurofibromas, ganglioneuromas, paragangliomas) were detected in 69.7%, malignant tumors (Ewing sarcomas, neuroblastomas, ganglioneuroblastomas, etc.) – in 30.3% of patients. The solution to treatment problems was determined by the characteristic localization of neurogenic tumors and the characteristics of their spread.
86 (96.6%) patients underwent surgery (most of them radically). Radical operations are mainly performed for benign neurogenic tumors; for malignant neoplasms, radical surgical interventions amounted to 54.2%, palliative ones – 45.8%. A typical version of the operation, involving removal of a mediastinal tumor, was performed in 81.4% of cases, combined - in 18.6% of cases. In case of malignant tumors, this ratio was 62.5 and 37.5%, respectively (7 patients), and typical operations were performed in combination with genic mediastinal tumors. Postoperative radiation or chemotherapy is indicated when there is doubt about the radicalism of the operation or when a significant extent of the tumor is detected. The prescription of additional radiation or chemotherapy is justified by information about the pathomorphosis of tumors and observations of tumor regression. The relapse rate after radical surgery was 4.8%. Continued tumor growth after palliative operations was detected in 72.8% of cases already at the end of the first year of observation. In this regard, the expediency of repeated (and multiple) surgical interventions for relapses of neurogenic mediastinal tumors is obvious - only the surgical method can achieve prolongation of life and elimination of painful local manifestations of the disease. The overall 3-5 year survival rate after reoperation was 70.8-38.6%, compared with 25.0-12.5% among patients treated conservatively.
Mesenchymal tumors (52 observations) are represented by various histological forms: vascular tumors - 17 (32.6%); fat – 15 (28.8%); fibroblastic – 6 (11.5%), fibrohistiocytic – 2 (3.8%); osteochondral – 5 (9.6%); mesenchymomas – 2 (2.8%), as well as tumors from skeletal muscles – 1 (1.9%); synovial sarcoma – 1 (1.9%); sarcomas of unknown origin – 3 (5.8%). Benign tumors accounted for 42.3%, malignant tumors – 57.7% of cases. Treatment of patients with mesenchymal tumors, especially malignant ones, in conditions of low effectiveness of chemoradiotherapy, is only surgical. Surgical interventions are very complex, which is usually due to the prevalence of the process. Surgical treatment was performed in 40 patients, conservative treatment in 12. In terms of additional treatment, only 7 patients received pre- or postoperative chemoradiotherapy. After surgical treatment in a group of 19 patients with malignant neoplasms, relapses were detected in 13 cases (68.4%) - including 9 out of 11 palliative and 4 out of 8 radical operations (81.8 and 50%, respectively). For relapses, repeated operations were performed; one of the patients was operated on three times, the other four times. Overall and relapse-free 5-year survival rates for benign mesenchymal tumors tend to 100%. In case of malignant – 1-3-5-year life expectancy was 75; 62.5; 48.6% after radical operations and 63.6; 27.2; 18.1% – after palliative treatment.
Thus, even with a widespread tumor process and with locoregional relapses, active surgical tactics should be used. Repeated (and multiple) operations, including those for palliative purposes, make it possible to once again prolong life. The aggressive local growth inherent in mesenchymal tumors and the tendency to relapse justify the advisability of further searching for combination treatment options using neoadjuvant or adjuvant chemoradiotherapy. Each component of treatment should be used in full, constantly developing and improving.
Extragonadal germ cell tumors (EGT). 114 patients with extragonadal germ cell tumors of the mediastinum were observed: men – 97 (85.1%), women – 17 (14.9%) aged from 14 to 72 years, average age – 26 years. Seminoma was diagnosed in 17.5%, non-seminoma – in 82.5% of cases. Among patients with non-seminoma VGOs, embryonal cancer, yolk sac tumors, choriocarcinomas, teratomas of varying degrees of maturity, and dermoid cysts were identified. General principles of diagnosis and treatment. If the level of tumor markers (AFP, hCG, LDH) increases without signs of testicular damage, computed tomography of the chest, abdominal cavity and retroperitoneal space is necessary. With morphological confirmation of the diagnosis of a malignant intrathoracic germ cell tumor, 4-6 courses of chemotherapy (VER, BP, etc. regimens), radiation therapy (ROD = 2-3 Gy; SOD - up to 60-70 Gy) are indicated. The full effect of treatment of patients with extragonadal seminomas is recorded in 80%, with non-seminomas - in 23% of cases. The relapse rate was higher in the group of extragonadal nonseminoma tumors (6.7 and 29.4%; p<0.05). If the seminoma tumor persists after induction chemotherapy, dynamic observation is indicated. If there is residual nonseminoma tumor after completion of induction therapy, surgical treatment is recommended. An increase in markers is an indication for second-line chemotherapy. Identification of viable germ cells in the residual tumor determines postoperative chemotherapy.
Lymphomas (with damage to the mediastinal lymph nodes) without other manifestations of the disease were identified in 129 patients. Hodgkin's lymphomas were diagnosed in 82 patients, non-Hodgkin's lymphomas - in 47 patients. The morphological diagnosis was established using surgical diagnostic methods. The surgical stage ended at the diagnostic stage. Subsequently, the patients were treated in accordance with the variant of lymphoma and a set of prognostic signs according to the chemotherapy and radiation therapy programs used at the Russian Cancer Research Center during the analyzed historical stages.
*
How does the da Vinci surgical robot work?
The operation of the da Vinci surgical robot is fully controlled by an experienced surgeon through small incisions no larger than 2 cm in size. An endoscope video camera inserted through one of the incisions transmits to the doctor a detailed three-dimensional image of the organ. As a result, the doctor can carefully plan the operation. The surgeon controls the instruments, which have 7 degrees of freedom of movement, thanks to EndoWrist technology and controls their movements inside the patient’s body remotely using special joysticks. Performing an operation using the da Vinci robot requires a highly qualified surgeon and special skills.