Ethanol
(ethyl alcohol, methyl carbinol, wine alcohol or alcohol, often colloquially simply “alcohol”) is a monohydric alcohol with the formula C2H5OH (empirical formula C2H6O), rational formula: CH3-CH2-OH, abbreviation
EtOH,
the second representative of the homologous series of monohydric alcohols, under standard conditions, a volatile, flammable, colorless, transparent liquid.
The active component of alcoholic beverages is a depressant - a psychoactive substance that depresses the human central nervous system.
Ethyl alcohol is also used as a fuel, as a solvent, as a filler in alcohol thermometers, and as a disinfectant (or as a component thereof).
Content
- 1 Preparation 1.1 Fermentation 1.1.1 Industrial production of alcohol from biological raw materials
- 1.1.2 Hydrolysis production
- 2.1 Physical properties
- 3.1 Fuel
- 5.1 Vehicle fleet running on ethanol
- 9.1 Etymology of the term "ethanol"
2. Methods of indicating the concentration of ethyl alcohol
In pharmacy practice, ethanol of varying concentrations is used. The initial concentration is 95%, 90%, 70% and 40% are also used. If the alcohol concentration is not indicated in the recipe, prepare 90%.
The concentration of ethyl alcohol is expressed as a percentage by volume (%v) or as a percentage by mass (%m).
The percentage by volume shows the content of anhydrous (absolute) alcohol in milliliters in 100 milliliters of alcohol solution at 20°C.
The percentage by weight shows the content of anhydrous (absolute) alcohol in grams in 100 grams of alcohol solution.
In the event that the alcohol concentration is simply indicated in %, it means percentage by volume (%v).
If there is a need to dilute strong ethyl alcohol or mix alcohol solutions of different concentrations, calculations are performed using:
- Dilution formulas;
- "Rules of the Cross".
- Alcoholometric tables of the State Fund XI;
Receipt
There are 2 main ways to produce ethanol - microbiological (alcoholic fermentation) and synthetic (ethylene hydration).
Fermentation
See also: Bioethanol § Fermentation
The method of producing ethanol, known since ancient times, is the alcoholic fermentation of organic products containing carbohydrates (grapes, fruits, etc.) under the action of yeast and bacterial enzymes. The processing of starch from potatoes, rice, and corn looks similar. The source of fuel alcohol is raw sugar produced from cane, etc. This reaction is quite complex, its result can be expressed by the equation:
C6H12O6 → 2C2H5OH + 2CO2
The solution obtained as a result of fermentation contains no more than 15% ethanol, since yeast is not viable in more concentrated solutions. The ethanol thus obtained needs to be purified and concentrated, usually by distillation.
To produce ethanol by this method, various strains of yeast of the species Saccharomyces cerevisiae are most often used, pre-treated sawdust and/or a solution obtained from them are used as a nutrient medium.
Industrial production of alcohol from biological raw materials
Modern industrial technology for producing ethyl alcohol from food raw materials includes the following stages:
- Preparation and grinding of starchy raw materials - grain (primarily rye, wheat), potatoes, corn, apples, etc.
- Fermentation. At this stage, the enzymatic breakdown of starch into fermentable sugars occurs. For these purposes, recombinant alpha-amylase preparations obtained by bioengineering are used - glucamylase, amylosubtilin.
- Fermentation. Due to the fermentation of sugars by yeast, alcohol accumulates in the mash.
- Bragorectification. It is carried out on accelerating columns.
Fermentation waste includes carbon dioxide, stillage, ether-aldehyde fraction, fusel alcohol and fusel oils.
The alcohol coming from the bragon rectification unit (BRU) is not anhydrous; the ethanol content in it is up to 95.6%. Depending on the content of foreign impurities in it, it is divided into the following categories:
- Alpha
- Lux
- Extra
- basis
- highest purification
- 1st grade
The productivity of a modern distillery is about 30,000-100,000 liters of alcohol per day.
Hydrolysis production
Main article: Hydrolysis alcohol
On an industrial scale, ethyl alcohol is produced from raw materials containing cellulose (wood, straw), which is preliminarily hydrolyzed. The resulting mixture of pentoses and hexoses is subjected to alcoholic fermentation. This technology was not widespread in the countries of Western Europe and America, but in the USSR (now in Russia) there was a developed industry of feed hydrolytic yeast and hydrolytic ethanol.
Ethylene hydration
In industry, along with the first method, ethylene hydration is used. Hydration can be carried out according to two schemes:
- direct hydration at a temperature of 300 °C, a pressure of 7 MPa, orthophosphoric acid deposited on silica gel, activated carbon or asbestos is used as a catalyst:
CH2 = CH2 + H2O → C2H5OH
- hydration through the stage of intermediate sulfuric acid ester, followed by its hydrolysis (at a temperature of 80-90 ° C and a pressure of 3.5 MPa):
CH2 = CH2 + H2SO4 → CH3CH2OSO2OH CH3CH2OSO2OH + H2O → CH3CH2OH + H2SO4
This reaction is complicated by the parallel reaction of diethyl ether formation.
Ethanol purification
Ethanol, produced by hydration of ethylene or fermentation, is a water-alcohol mixture containing impurities. For its industrial, food and pharmacopoeial use, purification is necessary. Fractional distillation produces ethanol with a concentration of about 95.6% (wt.); this azeotrope, inseparable by distillation, contains 4.4% water (wt.) and has a boiling point of 78.15 °C.
Distillation frees ethanol from both volatile and heavy fractions of organic substances (bottom residue).
Absolute alcohol
Absolute alcohol is ethyl alcohol containing virtually no water. It boils at 78.39 °C, while rectified spirit containing at least 4.43% water boils at 78.15 °C. It is obtained by distilling aqueous alcohol containing benzene and other methods, for example, the alcohol is treated with substances that react with water or absorb water, such as quicklime CaO or calcined copper sulfate CuSO4.
Ethyl alcohol as a solvent and extractant
- It is a good solvent for many alkaloids, glycosides, essential oils, resins and other substances that dissolve in water in small quantities.
- It penetrates cell walls much more difficult than water. By taking water away from proteins and mucous substances, alcohol can turn them into sediments that clog cell pores and thus impair diffusion. The lower the concentration of alcohol, the easier it penetrates into cells.
- The stronger the alcohol, the less possible hydrolytic processes are. Alcohol inactivates enzymes.
- It is a bactericidal environment. In extracts containing at least 20% alcohol, neither microorganisms nor molds develop.
- Pharmacologically indifferent. It has both local and general effects.
- Quite volatile.
- It is flammable, therefore, when working with it, established fire safety requirements must be observed.
- It is a limited product released by pharmaceutical production in accordance with the established procedure. However, in terms of cost, it is a quite affordable extractant.
So, ethyl alcohol is an extractant with an even wider range than water, and its extracting abilities depend on its concentration.
Properties
Physical properties
Under normal conditions, it is a colorless, easily mobile, volatile liquid with a characteristic odor and a sweetish-burning taste.
The density of ethyl alcohol is 0.7905 g/cm3 at 20 °C, it is lighter than water.
It is a good solvent for many organic substances and some inorganic salts.
The physical properties of absolute ethanol (100%) differ slightly from the properties of rectified alcohol with a concentration of 95.57%.
Their properties are almost identical, but the numerical values differ by 0.1-0.01%. Physical properties of ethanol
| Molecular mass | 46.069 a. eat. |
| Melting temperature | −114.15 °C |
| Boiling temperature | 78.39 °C |
| Critical point | 241 °C (at a pressure of 6.3 MPa) |
| Solubility | Miscible in arbitrary ratios with benzene, water, glycerin, diethyl ether, acetone, methanol, acetic acid, chloroform |
| Refractive index | Refractive index (for sodium D-line) 1.3611 (at 20 °C) (temperature coefficient of the refractive index −4.0⋅10−4/°C, almost constant in the temperature range 10-30 °C) |
| Standard enthalpy of formation ΔH | −234.8 kJ/mol (g) (at 298 K) |
| Standard entropy of formation S | 281.38 J/mol K (g) (at 298 K) |
| Standard molar heat capacity Cp | 1.197 J/mol K (g) (at 298 K) |
| Melting enthalpy Δ H pl | 4.81 kJ/mol |
| Enthalpy of boiling Δ H boil | 839.3 kJ/mol |
Reduction in the volume of the mixture when mixing ethanol with water at different mole fractions of ethanol in solution.
At a mole fraction of 40%, the volume reduction is maximum. A mixture by weight of 95.57% ethanol and 4.43% water is azeotropic, i.e. it does not separate during distillation, boils at normal pressure at a temperature of 78.174 ° C, while absolute ethanol has a higher boiling point of 78.39 °C.
Ethanol is mixed with water in an arbitrary ratio; when mixed, a significant, up to several percent, decrease in the volume of the mixture is observed relative to the initial total volume of pure substances, for example, when mixing 50 ml of ethanol with 50 ml of water, 97 ml of solution is formed. Mixing is also accompanied by some heating of the mixture.
Absolute ethanol solidifies at −114.5 °C. The melting point of ethanol-water mixtures decreases with increasing ethanol concentration in solution and reaches a minimum at a mass concentration of ethanol in water equal to 93.5% - the ethanol-water eutectic, which has a melting point of −118 °C. At low temperatures, below −20 °C, an aqueous solution of ethanol (96%) practically does not evaporate and turns into a viscous liquid. At −70 °C it becomes even more viscous and in fluidity resembles thick honey.
Chemical properties
Animation of a three-dimensional model of an ethanol molecule.
A typical representative of monohydric alcohols.
Flammable Highly flammable. With sufficient access to air, it burns (due to its oxygen) with a light bluish flame, forming terminal oxidation products - carbon dioxide and water:
C2H5OH + 3O2 → 2CO2 + 3H2O
This reaction proceeds even more vigorously in an atmosphere of pure oxygen.
Under certain conditions (temperature, pressure, catalysts), controlled oxidation (both with elemental oxygen and many other oxidizing agents) to acetaldehyde, acetic acid, oxalic acid and some other products is possible, for example:
3C2H5OH + K2Cr2O7 + 4H2SO4 → 3CH3CHO + K2SO4 + Cr2(SO4)3 + 7H2O
It has mild acidic properties, in particular, like acids, it interacts with alkali metals, as well as magnesium, aluminum and their hydrides, releasing hydrogen and forming salt-like ethylates, which are typical representatives of alcoholates:
2C2H5OH + 2K → 2C2H5OK + H2 C2H5OH + NaH → C2H5ONa + H2
Reacts reversibly with carboxylic and some inorganic oxygen-containing acids to form esters:
C2H5OH + RCOOH ⇄ RCOOC2H5 + H2O C2H5OH + HNO2 ⇄ C2H5ONO + H2O
With hydrogen halides (HCl, HBr, HI) it enters into reversible nucleophilic substitution reactions:
C2H5OH + HX ⇄ C2H5X + H2O
Without catalysts, the reaction with HCl is relatively slow; much faster - in the presence of zinc chloride and some other Lewis acids.
Instead of hydrogen halides, phosphorus halides and halide oxides, thionyl chloride and some other reagents can be used to replace the hydroxyl group with a halogen, for example:
3C2H5OH + PCl3 → 3C2H5Cl + H3PO3
Ethanol itself also has nucleophilic properties. In particular, it binds relatively easily at activated multiple bonds, for example:
C2H5OH + CH2 = CHCN → C2H5OCH2CH2CN
reacts with aldehydes to form hemiacetals and acetals:
C2H5OH + RCHO → RCH(OH)OC2H5 C2H5OH + RCH(OH)OC2H5 → RCH(OC2H5)2 + H2O
When heated moderately (not above 120 °C) with concentrated sulfuric acid or other acidic water-removing agents, diethyl ether is formed:
2C2H5OH → C2H5OC2H5
With stronger heating with sulfuric acid, as well as when passing vapors over aluminum oxide heated to 350÷500 °C, deeper dehydration occurs. This produces ethylene:
C2H5OH → CH2 = CH2 + H2O
When using catalysts containing, along with aluminum oxide, highly dispersed silver and other components, the dehydration process can be combined with the controlled oxidation of ethylene with elemental oxygen, as a result of which it is possible to implement a one-stage process for the production of ethylene oxide with satisfactory yield:
2C2H5OH + O2 → 2C2H4O + 2H2O
In the presence of a catalyst containing oxides of aluminum, silicon, zinc and magnesium, it undergoes a series of complex transformations with the formation of butadiene as the main product (Lebedev reaction):
2C2H5OH → CH2 = CH – CH = CH2 + 2H2O + O2
In 1932, based on this reaction, the world's first large-scale production of synthetic rubber was organized in the USSR.
In a slightly alkaline environment it forms iodoform:
C2H5OHA + 4I2 + 6NaHCO3 → CHI3 + HCOONa + 5NaI + 5H2O + 6CO2
This reaction is of some importance for the qualitative and quantitative determination of ethanol in the absence of other substances that give a similar reaction.
Fire properties
Highly flammable colorless liquid; saturated vapor pressure, kPa: log p = 7.81158-1918.508/(252.125+t) at temperatures from −31 to 78°C; heat of combustion - 1408 kJ/mol; heat of formation −239.4 kJ/mol; flash point 13°C (in a closed crucible), 16°C (in an open crucible); ignition temperature 18°C; auto-ignition temperature 400°C; concentration limits of flame propagation 3.6–17.7% of volume; temperature limits for flame propagation: lower 11°C, upper 41°C; minimum phlegmatizing concentration, % volume: CO2 - 29.5, H2O - 35.7, N2 - 46; maximum explosion pressure 682 kPa; maximum rate of pressure rise 15.8 MPa/s; burnout rate 0.037 kg/(m2•s); maximum normal flame propagation speed - 0.556 m/s; minimum ignition energy - 0.246 MJ; The minimum explosive oxygen content is 11.1% volume.
3. Features of dilution of ethyl alcohol, calculations
Dilution formulas
Dilution by volume.
If dilution is carried out with water, calculations are made according to formula (1):
(1), where
X – volume of strong alcohol (ml);
V – volume of alcohol of the desired strength (ml);
b – alcohol concentration of the desired strength, %(v);
a is the concentration of strong alcohol, %(v);
If strong alcohol is diluted with alcohol of a lower concentration, calculations are made using formula (2):
(2) , where
с – concentration of weak alcohol, % (v)
Since volume depends on temperature, liquids are measured at a standard temperature (20±2°C).
Dilution by weight.
If dilution is carried out with water, calculations are made according to formula (3):
(3),where
X – mass of strong alcohol (g);
P – mass of alcohol of the desired strength (g);
b – alcohol concentration of the desired strength, % (m);
a – concentration of strong alcohol, % (m).
If strong alcohol is diluted with alcohol of a lower concentration, calculations are made using the formula:
X = P (4) , where
с – concentration of weak alcohol, %(m).
"Rule of the Cross"
If it is required to prepare a solution of concentration b% by mixing solutions of concentration a% and c%, provided that a>b>c, the question arises in what ratio of a% and c% solutions should be used? Denoting these volumes by x and y, we obtain the equation:
ax + cy = b (x + y) , hence
Equating the corresponding terms of the relations, we get:
x = b – c
y = a - b
Therefore, the condition of the problem and the result obtained can be written as follows:
a b – c (5)
b
c a - b
In the event that the alcohol concentration is expressed as a percentage by volume, the dimension of the values obtained by arithmetic subtraction is expressed in volume units (ml). If the alcohol concentration is expressed as a percentage by mass, then the dimension of the values is in units of mass (g).
Alcoholometer tables
The XI edition of the State Fund of the USSR includes five alcoholmetric tables that allow you to quickly make the necessary calculations for diluting alcohol. (For more details about these tables, read the abstract, which you can download from the link after the text)
Application
Fuel
The first to use ethanol as a motor fuel was Henry Ford, who in 1880 created the first car running on ethanol. The possibility of using alcohols as motor fuel was also shown in 1902, when more than 70 carburetor engines running on ethanol and ethanol-gasoline mixtures were exhibited at a competition in Paris.
Ethanol can be used as fuel, including for rocket engines (for example, 75% aqueous ethanol was used as fuel in the world's first serial ballistic missile - the German V-2 and early Soviet rockets designed by Korolev - from R -1 to R-5), internal combustion engines, household, camping and laboratory heating devices (so-called “spirit lamps”), heating pads for tourists and military personnel (catalytic auto-oxidation on a platinum catalyst). It is used to a limited extent (due to its hygroscopicity) in a mixture with classic petroleum liquid fuels. It is used to produce high-quality fuel and a component of gasoline - ethyl tert-butyl ether, which is more independent of fossil organic matter than MTBE.
Chemical industry
- serves as a raw material for the production of many chemicals, such as acetaldehyde, diethyl ether, tetraethyl lead, acetic acid, chloroform, ethyl acetate, ethylene, etc.;
- widely used as a solvent (in the paint and varnish industry, in the production of household chemicals and many other areas);
- is a component of antifreeze and windshield washers;
- In household chemicals, ethanol is used in cleaning products and detergents, especially for the care of glass and plumbing. It is a solvent for repellents.
Medicine
In medicine, ethyl alcohol is primarily used as a solvent, extractant and antiseptic
See also: Medical antiseptic solution
- In terms of its action, ethyl alcohol can be classified as an antiseptic;
- as a disinfectant and drying agent, externally;
- the drying and tanning properties of 97% ethyl alcohol are used to treat the surgical field or in some techniques for treating the surgeon’s hands;
- solvent for medicines, for the preparation of tinctures, extracts from plant materials, etc.;
- preservative for tinctures and extracts (minimum concentration 18%);
- defoamer when supplying oxygen, artificial ventilation;
- in warm compresses;
- for physical cooling during fever (for rubbing);
- component of general anesthesia in situations of drug shortage;
- as an antifoam agent for pulmonary edema in the form of inhalation of a 33% solution;
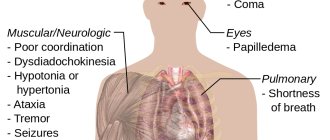
- ethanol is an antidote for poisoning with certain toxic alcohols, such as methanol and ethylene glycol. Its action is due to the fact that the enzyme alcohol dehydrogenase, in the presence of several substrates (for example, methanol and ethanol), carries out only competitive oxidation, due to which, after timely
(almost immediate, following methanol/ethylene glycol) intake of ethanol, the current concentration of toxic metabolites decreases (for methanol - formaldehyde and formic acid, for ethylene glycol - oxalic acid).
Perfumes and cosmetics
It is a universal solvent for various substances and the main component of perfumes, colognes, aerosols, etc. It is part of a variety of products, including toothpastes, shampoos, shower products, etc.
Food industry
Along with water, it is the main component of alcoholic beverages (vodka, wine, gin, beer, etc.). It is also found in small quantities in a number of drinks obtained by fermentation, but not classified as alcoholic (kefir, kvass, kumiss, non-alcoholic beer, etc.). The ethanol content in fresh kefir is negligible (0.12%), but if it has stood for a long time, especially in a warm place, it can reach 1%. Kumis contains 1-3% ethanol (in strong ethanol up to 4.5%), kvass - from 0.5 to 1.2%.
Solvent for food flavorings. Can be used as a preservative for bakery products, as well as in the confectionery industry.
Registered as a food additive E1510
.
The energy value of ethanol is 7.1 kcal/g.
Alcohol recipe in Latin: step-by-step instructions
A recipe for alcohol in Latin begins, like any other recipe in Latin, with the word Recipe (abbreviated in all recipes - Rp.), which means “take.”
Rp.
Further, after the colon, it is necessary to indicate the dosage form of the substance in the genitive case and in the singular. In our case, it will always be a solution, since alcohol is used only in solution. In Latin it looks like this: Solutionis - (abbreviated - Sol.). Read more about the recipe for the solution in Latin.
Rp.: Sol.
Then we write the word alcohol in Latin in the singular and in the genitive case: Spiritus aethylici. Yes, it does not change in the genitive case.
Rp.: Sol. Spiritus aethylici
After which you need to indicate the percentage of our alcohol and the volume in milliliters, for example, let’s take a solution of 70% - 200 ml.
Rp.: Sol. Spiritus aethylici 70% – 200 ml
On a new line we write Da tales doses numero (abbreviated as Dtd N), which means “give out in this quantity” and indicate how many (for example, 12 pieces) and in what (in bottles - in flacconis - in flacc.) you need to give out: Dtd N 12 in flacc. It is not necessary and even undesirable to indicate “in bottles”; you will learn more about this in the article recipe for bottles in Latin.
Rp.: Sol. Spiritus aethylici 70% – 200 ml Dtd N 12
On a new line we write Signa (abbreviated S.), which translates as “designate”, put a colon and write how to use our alcohol to the patient.
Rp.: Sol. Spiritus aethylici 70% – 200 ml Dtd N 12 S. Use for disinfection
Alcohol can also be prescribed as part of other medicines, for example:
Rp.: Lotionis Zinci spirituosae 100.0 (zinc-alcohol lotion) DS for wiping chafed skin areas
5. Dispensing ethyl alcohol from pharmacies
Alcohol is dispensed from pharmacies to outpatients in accordance with the requirements of Order No. 110 of 2007 of the Ministry of Health of the Russian Federation:
- in its pure form - up to 50 grams according to recipes labeled “For applying compresses” (indicating the required dilution) or “For treating the skin”;
- in a mixture with other ingredients in the individual manufacture of medicinal products - no more than 50 grams;
- for patients with a chronic course of the disease - up to 100 grams according to prescriptions with the inscription “For special purposes”, separately signed by the doctor and the seal of the medical institution “For prescriptions”.
Recipes for making wine at home
Wine is a refined and pleasant-tasting alcoholic drink. In addition, its moderate consumption is beneficial for the health of the entire body. An alcoholic masterpiece can be prepared not only from fresh grapes. It is easy to make at home even in winter.
To do this you will need:
- 4 liters of regular grape juice;
- 1 kg sugar;
- 1 liter of alcohol.
First, mix the juice with sugar and put it on low heat. When the sweet ingredient has dissolved, remove the liquid from the stove and cool completely. Then you need to install a water seal, placing the wine stock in a warm place for fermentation for two weeks
It is very important to add sugar once every few days and stir it thoroughly.
After the expiration date, you must pass the drink through a sieve or cheesecloth. Add alcohol to it in an amount not exceeding 20% of the total volume of liquid. The resulting mixture should be placed in a dark, cool place for 14 days. It is easy to get rid of sediment accumulated at the bottom using a special hose. This alcoholic drink must be infused for five months.
As a base, instead of classic grape juice, you can use a raspberry or cherry substitute. The resulting wine will have an original and pleasant taste.
Premises requirements
When designing alcohol enterprises, it is necessary to combine in one building all departments and production workshops, which are interconnected by one technological process. Mechanical repair shops, material warehouses, etc. are located in a common fenced area, but separate from the main building. As an option, separate them from explosive production with blank fire walls.
A fermentation department and a room for preparing an aromatic product are designed separately. It is necessary to install supply and exhaust ventilation there. The fermentation process is prohibited in semi-basements and basements.
All technological processes are monitored by operators whose workplaces are located in the equipment room.
Every year the production workshops are painted and whitewashed. The walls are covered with moisture-resistant paint or lined with glazed tiles to a height of 1.8 m. Floors in rooms where acids and alkalis are used must be impervious to accidental contact with aggressive substances. For auxiliary premises, it is allowed to use water-based paint to cover the walls.
The premises must be dry and heated. The minimum permissible air temperature is 8 degrees Celsius. All necessary communication systems have been installed. Non-food substances are not stored in food compartments. There must be fire extinguishing means on the territory of the enterprise: sand, fire extinguishers, asbestos blanket.
In general, the work areas are distributed as follows:
- a production workshop consisting of several departments, including a hardware room;
- offices;
- office premises (the number of bathrooms is determined based on the number of people per shift);
- warehouses (for storing finished products and raw materials);
- a dining room or a specially designated room for eating (it is better to make it a separate building) and so on.
The equipment can be so tall that it takes up two floors at once. The total area of the premises will be from 150 m2.
Bibliography
- Pharmaceutical technology. Technology of dosage forms: textbook. for students higher textbook institutions / [I.I. Krasnyuk, S.A. Valevko, G.V. Mikhailova, etc.] ; edited by I.I. Krasknyuk, G.V. Mikhailova. – 3rd ed., revised. and additional – M.: Publishing House, 2007. – 592 p.
- State Pharmacopoeia of the USSR. – 11th ed. – T.1. – M.: Medicine, 1987. – 336 p. T.2. – M.: Medicine, 1987. – 400 p.
- XII State Pharmacopoeia of the Russian Federation / Publishing House “Scientific Center for Expertise of Medicinal Products”, 2008. – 704 pp.: ill.
- Orders of the Ministry of Health of the Russian Federation No. 308 of 1997, 214 of 1997, 305 of 1997, 309 of 1997, 110 of 2007.
All formulas are given in the document, which you can download from the link >>>
There is also a presentation on this abstract >>>
What you need to know about wine filtration?
- Filtration is important. Through the filtering process, wine becomes not only clean and transparent. During preparation, dust, fungi, seeds and other small particles will get into the drink. After all, according to the rules, grapes and other fruits are not washed, preserving natural yeast. If filtering is not carried out, the wine may become unfit for consumption.
- Winemakers claim that a place for filtration can be found at any stage of preparation. Then the question needs to be posed differently: what kind of wine will be obtained after passing through the filter at a certain stage?
- There is coarse, fine, acute and final warranty filtration. Experienced winemakers filter the result of their labor using special devices.
- When straining wine, you need to monitor the amount of sediment. It doesn't always require as many steps as described in the recipe. First you need to learn how to strain homemade wine at home.
Can alcohol be diluted or mixed with carbonated or distilled water?
Worth knowing:
- Although it is written everywhere that distilled water should not be used for drinking, alcohol is diluted with distillate quite often. The main harm of distilled water is the absence of the bulk of useful substances in it, which contributes to the leaching, rather than saturation, of useful substances in the body.
- Distilled water has an unpleasant odor and specific taste, so it should not be used. There are no other restrictions on diluting alcohol with it. Although, if a person does not have a particularly sensitive taste, he may not feel it. But some fans, on the contrary, prefer to use exclusively distilled water, since without additional additives, vodka with it turns out softer and more pleasant.
- Alcohol is not diluted with carbonated water; the desired effect - a tasty, pleasant drink cannot be achieved. Carbon dioxide increases the absorption of alcohol and irritates the walls of the stomach. In order to speed up the absorption of alcohol, it is better to simply drink the finished vodka with a carbonated drink.
Taste pleasure!