Pharmacological properties of the drug Glatiramer acetate
Acetic acid salt of synthetic polypeptides consisting of L-glutamine amino acid, L-alanine, L-tyrosine and L-lysine, and is similar in chemical structure to myelin basic protein. Modifies the immune response and affects immunocompetent cells. Competes with myelin basic protein and oligodendrocyte glycoprotein, as well as proteolipid protein for binding to major histocompatibility complex class II molecules on the surface of cells bearing antigens. Stimulates the formation of antigen-specific T-lymphocytes. Blocks myelin-specific autoimmune reactions that cause destruction of the myelin sheath of nerve fibers (demyelination) in multiple sclerosis. When treating patients with relapsing-remitting multiple sclerosis, it reduces the frequency of exacerbations and slows down the rate of onset of irreversible neurological disorders. Most of glatiramer acetate administered subcutaneously is hydrolyzed locally, a certain amount enters the lymphatic system, and a small part enters the systemic circulation unchanged. The possibility of glatiramer acetate passing into human breast milk has not been studied.
Currently, multiple sclerosis course-modifying drugs (MCDs) are actively used in the widespread practice of neurologists. First-choice drugs include beta-interferons and glatiramer acetate. The basis for their introduction was the results of large-scale randomized clinical trials, which showed that the administration of these immunomodulators during multiple sclerosis (MS) with exacerbations can reliably and significantly reduce their frequency, which is accompanied by a significant reduction in focal brain damage in patients according to magnetic resonance imaging (MRI). ) [9]. The rate of progression of disability, associated primarily with the neurodegenerative process, decreases to a lesser extent, but even in this case there are a number of significant differences from placebo. These clinical studies had a relatively short duration - up to 2-3 years, so the question is increasingly being asked what is the effectiveness of long-term courses of DMT, since people with MS have been suffering for decades [3, 9]. Three aspects are discussed: effectiveness, safety and tolerability of long-term courses. It is not yet clear exactly how long the effect of the drug lasts, whether there are “addiction” and “escape” phenomena, whether it is possible to suspend/discontinue the course with a good clinical result, for example, in the absence of exacerbations and progression of the disease for 3, 5 or more years. This is especially relevant due to the fact that one of the common reasons for decreased adherence to therapy lasting more than 5 years is the assumption of a “cure”, a complete stop of the pathological process, which leads to termination of the course [4].
Considering the high cost and specificity of this therapy, the use of injectable forms that affect the quality of life of patients, prospective observations of patients receiving a specific drug for a long time are of great importance. In practice, conducting such studies is extremely difficult for organizational, medical and socio-economic reasons. To analyze the results of long-term use of DMTRS, it is necessary that a large number of patients are observed for a long time in a certain neurological center, there is an opportunity to constantly receive the drug, and detailed clinical information is available for analysis.
This report analyzes the results of open observation of MS patients receiving glatiramer acetate (Copaxone) for a long time at the Moscow City Center for Multiple Sclerosis (MCMS) at the Moscow City Clinical Hospital No. 11. The study was conducted jointly with the Department of Neurology and Neurosurgery of the Russian National Research Medical University (RNRMU) named after. N.I. Pirogov. The MGCRS was created in 1998 and almost all patients with this diagnosis are regularly observed there. A register of patients has been created at the center.
Previously, we published an analysis of 3- and 5-year experience in using PMTRS [1, 2].
The purpose of this study was to analyze the results of using glatiramer acetate over a 10-year period.
Material and methods
As a result of targeted purchases by the Department of Health of the Moscow Government, since 2001, Moscow residents with MS have the opportunity to receive DMT for a long time free of charge. Since 2005, patients began to receive drugs through additional benefits, in recent years - as part of the implementation of the federal program “Seven Nosologies”. These patients have the opportunity to be regularly observed by neurologists, most of whom are employees of the Department of Neurology and Neurosurgery of the Russian National Research Medical University (formerly the Russian State Medical University) named after. N.I. Pirogov. At the end of 2001, 155 patients began receiving Copaxone through the Moscow Department of Health. The criteria for prescribing the drug then were reliable MS according to the criteria of C. Pozer et al. [10] with a remitting course, the presence of at least 2 exacerbations of the disease over the past 2 years, as well as the severity of the disease on the Kurtzke scale (EDSS, Expanded Disability Status Scale) from 1.5 to 5.0 points. By decision of a special commission of this department, the drugs were prescribed in the absence of any other neurological disease causing the patient’s symptoms, in the absence of pronounced psychopathological changes, and with the exception of pregnancy and lactation in women. At the same time, through various channels related to federal procurement, including as part of a post-marketing clinical trial, about 50 more patients began to receive glatiramer acetate, who were then registered and monitored at the Moscow City Center for Radiological Research, continuing to receive the drug at their place of residence.
Throughout the entire period of treatment, patients were required to undergo a comprehensive examination once every 3 months at the Moscow City Center for Radiological Research in pursuance of the orders of the Moscow Department of Health, and then the recommendations of the All-Russian Society of Neurologists and the corresponding technology approved by Roszdravnadzor. During the examinations, the frequency of exacerbations, their severity and duration, the rate of increase in confirmed (sustained for at least 3 months) neurological deficit on the EDSS scale, as well as the severity of adverse reactions were recorded.
The course of treatment with Copaxone was carried out according to the standard regimen at the recommended dosage of 20 mg subcutaneously every day. Clinical data were collected according to a unified scheme every 3 months and recorded in the outpatient chart.
Statistical data processing was carried out using the Statistica software package. In all types of statistical analysis, differences were considered statistically significant at a significance level of p
<0,05.
results
The results below are given in comparison with data from a 5-year follow-up.
In 2011, the clinical condition and course of MS was analyzed in all patients who continued to continuously receive the drug. If the drug was discontinued for various reasons, it became impossible to monitor the effect of Copaxone on the course of MS, since most patients then received other drugs from the DMT group.
As a result, without interruptions or replacements for the entire 10 years, 67 patients continued to receive Copaxone in 2011 from those who began receiving the drug through the Department of Health in 2001. Another 15 patients began receiving the drug through the federal line, and then went under the supervision of the MGTSRS. Thus, the initial sample consisted of 82 patients who had received the drug for 10 years and had never been treated for MS by other methods. Of these 82 patients, 6 women had a course interruption of varying duration due to pregnancy, so they were excluded from the analysis. In another 2 cases, medical information was insufficient, so they were also excluded from the analysis. As a result, the analysis included data from 74 patients (55 women - 74.3% and 19 men - 25.7%). At the start of the course in 2001, their average age was 32.9±0.8 years and the duration of MS was 5.37±0.44 years (Table 1).
Before the start of the course, these patients had a high frequency of exacerbations (on average 2.15±0.06 per year) with a low EDSS level - 2.1±0.1 points. Among them, 6 (8.1%) patients had 1 exacerbation per year, the majority - 51 (68.9%) patients - 2 exacerbations per year, 17 (23%) - 3 or more exacerbations per year. Depending on the level of EDSS, a group of patients can be distinguished with a score of up to 2 points - 31 (41.9%) patients, from 2 to 2.5 - 23 (31.1%) patients and 3 or more points - 20 (27%) sick. Thus, patients with actively relapsing-remitting and non-severe MS at the time of the start of the course received Copaxone without a break for 10 years.
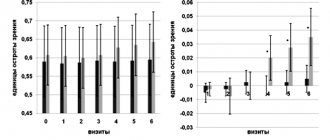
During treatment with Copaxone, a decrease in the frequency of exacerbations of the disease was noted. In the 1st year of therapy, all 74 patients had only 4 exacerbations, in the 2nd year - 6, in the 3rd-10th years - from 8 to 15 exacerbations. Thus, a low frequency of exacerbations was recorded throughout all 10 years, with a slight increase in years 5-7. The average annual frequency of exacerbations was significantly lower for all 10 years than before the start of the course, while being minimal in the first 3 years of treatment - from 0.07 to 0.11 on average per year, with a slight increase to 0.16-0.22 per year year for the 4th-7th years of treatment (Fig. 1).
Figure 1. Dynamics of the average annual frequency of exacerbations (along the y-axis) during 10 years of Copaxone therapy.
All indicators are significantly lower than before treatment: * - p
<0.05. Over 10 years, there were no exacerbations in 20 (27.0%) patients, 28 (37.8%) had only 1 exacerbation. Thus, regular administration of the drug practically freed 64.8% of patients from exacerbations of MS. 7 patients had 4 exacerbations and 1 had 5, i.e. exacerbations with a frequency of 1 in 2 years persisted only in 10.8% of patients.
The severity of the patients’ condition, assessed by the EDSS level, even decreased slightly in the first years, starting to increase from the 8th year of treatment and reaching a significant difference from the average before treatment only in the 10th year of therapy (Fig. 2).
Figure 2. Dynamics of the average EDSS level in points (ordinate) during treatment with Copaxone for 10 years (x-axis).
Only in the 10th year did the indicator become significantly higher than before the start of therapy: * - p
<0.05. There was no progression of EDSS in 35 (47.3%) patients. A minimum progression of 0.5 points was noted in 18 (24.3%) patients, i.e. 71.6% of MS patients had virtually no progression in the severity of the condition over 10 years of Copaxone therapy. In 6 patients the index increased by 1 and in 3 by 1.5 points. Only in 12 (16.2%) patients over these 10 years, the EDSS score increased by 2 or more points.
This positive dynamics is explained by the peculiarity of the sample, which was characterized by a remitting course throughout therapy with a minimal number of exacerbations, which made it possible to receive Copaxone for such a long time without changing the treatment regimen.
The previous analysis of a continuous course over 5 years was carried out in 91 patients, the majority of them (76.9%) were also women, the average age of the patients was 36.5±1.0 years and the duration of MS at the start of the course was 8.1 ±0.8 years, i.e. this group was significantly older and had a longer duration of disease (see Table 1). In both groups, during treatment with Copaxone, a significant ( p
<0.05) reduction in the frequency of exacerbations, which remained with slight fluctuations at the level of 0.3-0.5 on average per year. Over 5 years, 29 (31.9%) of 91 had no exacerbations at all, i.e. in ⅓ of patients. Only in the 5th year was there a significant increase in the average EDSS.
In 57 (62.6%) patients from this group, no progression of MS severity was observed over 5 years, and in 12 (13.2%) patients - only by 0.5 points, i.e. immaterial. Thus, in 75.8% of patients who constantly received Copaxone for 5 years, there was virtually no progression in the severity of the disease, which confirmed the neuroprotective effect of the drug [2]. Significant progression of MS severity by more than 1 point was observed only in 6 (6.6%) patients constantly receiving the drug. At the same time, it should be noted that the transition to secondary progression of MS, in which rapid progression of MS severity is expected, was an indication for discontinuation (replacement) of the drug. When comparing these groups of patients, it can be noted that patients who received Copaxone for 10 years were significantly younger at the beginning of the course and had shorter duration and severity of MS.
The drug was well tolerated over 10 years. Over the 10 years of the course, all patients fully adapted to daily injections, without experiencing any problems with organizing their lives. The only significant problem was local reactions, which were observed in 16 (21.6%) patients at different times, but only 3 (4%) patients had the development of lipodystrophy. In no case did these adverse reactions lead to discontinuation of the drug, so it is difficult to assess the overall tolerability of the drug in this sample. Mild “systemic” vascular reactions, clinically close to a “panic attack,” were noted only in 5 (6.8%) patients from this group and also did not lead to discontinuation of the course.
To analyze the characteristics of the drug's effect depending on the severity of MS at the beginning of the course, patients were divided into 2 subgroups - with an EDSS level before treatment of less than 3 points (54 patients) and an indicator of 3 or more points (20 patients).
Before the start of therapy, more severe patients were older and had a longer duration of MS, although the latter difference did not reach a statistically significant level (Table 2).
These subgroups did not differ in the average annual frequency of exacerbations before treatment. The dynamics of the average annual frequency of exacerbations in the subgroups during treatment are also similar, with the exception of the 3rd, 5th and 7th years of treatment, when with an initially higher EDSS score there were significantly more exacerbations, which may be a feature of the sample. In milder patients, at the beginning of the course there was a decrease in the average EDSS level, but from the 8th year it became significantly higher than it was before treatment. In the second subgroup, an increase in the severity of MS was noted from the 4th-5th year. Thus, the drug had a positive therapeutic effect regardless of the initial EDSS indicator.
Discussion
The positive therapeutic effect of glatiramer acetate was observed throughout all 10 years of therapy, which led to a significant decrease in the frequency of exacerbations of the disease. This reduction (on average approximately 30 times) was achieved already in the 1st year of treatment and turned out to be stable throughout the entire 10 years of use of the drug. Overall, for the entire period, the rate of exacerbations for the entire group was only 0.14 per year. Over 10 years, 64.8% of patients had no exacerbations at all or only one. This indicates the persistence of the anti-inflammatory and immunomodulatory effects of the drug. During therapy, stabilization of MS severity indicators according to EDSS was also noted. An increase in the disability indicator was noted after 7 years of treatment and it reached a significant level of difference compared to the data before treatment only in the 10th year of therapy. It was noted that the drug had a positive effect regardless of the initial EDSS score (less or more than 3 points).
It is known that the main effect of all first-choice drugs from the DMT group is associated with immunomodulation and a decrease in inflammatory activity, although data indicating their neuroprotective effect is also accumulating. This also applies to Copaxone, which is confirmed by our data on long-term stabilization of the severity of MS with constant use of the drug. It should be noted, however, that our sample did not include patients in whom the drug was discontinued due to transition to secondary progression with an increase in EDSS. Analysis of these cases is planned to be carried out in the future.
Data from a 10-year observation of patients receiving DMTRS in everyday neurological practice indicate a persistent positive effect in those patients who continued to receive Copaxone without interruption. The results obtained are generally close to the results of the analysis of 3- and 5-year experience of use [1, 2], although this sample of patients has its own characteristics. It is distinguished by its younger age and shorter duration of MS, i.e. in these cases, it was possible to prescribe the drug earlier, which could lead to its high effectiveness.
There was no statistically significant escape phenomenon if there was an initial decrease in the frequency of exacerbations, although epidemiological data indicate a consistent decrease in the frequency of exacerbations as the natural history of MS progresses and secondary progression of MS without exacerbations approaches. In this regard, it is especially important to start the course of treatment early at the stage of MS, when there is a high frequency of disabling exacerbations and the progression of irreversible neurological disorders is less pronounced.
Our results generally correspond to the literature data, although the number of long-term observations carried out like ours is very small. The average number of exacerbations in the 5th year of the open-label study of Copaxone in the USA [11] averaged only 0.16 per year; 25% of patients did not have a single exacerbation during follow-up. In 75.8% of patients who constantly took Copaxone, there was no progression in the severity of the disease over 5 years. In a 10-year prospective follow-up of patients continuing treatment after a clinical trial of Copaxone, exacerbation rates decreased to 0.23 at 6 years and 0.2 at 8 years (approximately 1 exacerbation per 5 years). The reduction in exacerbation rates persisted after 10 years, with 62% of patients having stable EDSS scores [5]. A significant difference between the two groups (receiving the drug from the very beginning and switching to it from placebo after 30 months) was in the proportion of patients with a stable EDSS score (65.3 and 50.4%, respectively, p
=0.026). Probably, this effect can be explained by the delay in the start of treatment with Copaxone by 30 months in the second group of patients [6, 7], which once again confirms the rule for all DMTs - prescribe as early as possible, since “you can’t get back what’s lost.”
Thus, in cases where a positive effect is noted, the drug should be used for as long as possible. With proper management of patients, in the vast majority of cases, side effects should be stopped and are not the main reason for discontinuation of the drug, as indicated by domestic and international experience [8].
The main result of the study is that with continuous 10-year use of Copaxone, 64.8% of patients had no more than 1 exacerbation, and in 71.6% of patients with MS there was no progression of the severity of the condition or was minimal - up to 1 point on the EDSS scale, which allows us to talk about control over the activity of the pathological process. Despite the obvious positive clinical effect and the minimal number of exacerbations, the severity of MS nevertheless continues to increase over time, reaching significant differences from the level before treatment by the 8th year (in milder patients), and for the entire group by the 10th year of the course . This makes it necessary to search for new, even more effective methods of pathogenetic treatment, although for a subgroup of young patients with a short duration of MS, the appointment of Copaxone can be considered optimal. The effect of this drug is sufficient to significantly reduce the activity of relapsing-remitting MS over 10 years. But due to the danger of developing lipodystrophies during regular injections, the problem of developing drugs with similar clinical efficacy, but a more convenient dosage form remains open.
Side effects of the drug Glatiramer acetate
From the cardiovascular system: heaviness and chest pain, palpitations, tachycardia, arrhythmia, vasodilation, increased blood pressure. From the gastrointestinal tract: anorexia, nausea, vomiting, diarrhea, difficulty swallowing, thrush. From the nervous system: asthenia, migraine, anxiety, syncope, tremor. From the senses: vertigo, nystagmus, blurred vision. From the respiratory system: shortness of breath, heaviness in the chest, difficulty breathing, flu-like syndrome (chills, hyperthermia, muscle pain), laryngospasm, bronchitis, hyperventilation. From the genitourinary system: peripheral edema, dysmenorrhea, hematuria, decreased potency, questionable Papanicolaou test results. Allergic reactions: skin rashes (including urticaria, urticaria, ecchymosis), angioedema of the face. Local reactions: hyperemia, swelling, itching of the skin, swelling and pain at the injection site. Other: arthralgia, lymphadenopathy, back pain, sweating.