Sodium iodide
- 7681-82-5 Y
- 13517-06-1 (dihydrate) N
- Interactive image
- CHEBI: 33167 Y
- ChEMBL1644695 N
- 5048 Y
- 5238
- WB6475000
- F5WR8N145C Y
- DTXSID2041125
- InChI = 1S / HI.Na / h1H; /q; +1/p-1Y
Key: FVAUCKIRQBBSSJ-UHFFFAOYSA-M Y
- InChI = 1/HI.Na/h1H; /q; +1/p-1
Key: FVAUCKIRQBBSSJ-REWHXWOFAL
- . [I-]
D )1.93 (300 nm) 1.774 (589 nm) 1.71 (10 µm) [7] Structure [8] Crystal structure Halite, cF8 Space group Fm 3 m, No. 225 Lattice constant a
= 0.6462 nm Unit formula ( Z
)4 Coordination geometry Octahedral Thermochemistry [9] Heat capacity ( C
)52.1 J mol −1 K −1 Standard molar entropy ( S
o 298 )98.5 J mol −1 K −1 Std formation enthalpy (Δ F H
⦵ 298 )−287.8 kJ mol −1 Gibbs free energy (Δ f G
˚)−286.1 kJ mol −1HazardsPrincipal HazardsIrritating, may cause harm to the unborn childSafety Data Sheet[1]GHS PictogramsGHS Signal Word Hazard Hazard Statements GHSH315, H319, H400 Precautionary Statements GHSP273, P305 + 351 + 338 [10]NFPA 704 (fire diamond ) 1
0
1 flash point Non-flammable Related compounds Other anions Sodium fluoride Sodium chloride Sodium bromide Sodium astatide Other cations Lithium iodide Potassium iodide Rubidium iodide Cesium iodide France iodide Unless otherwise noted, data are for materials in their standard state (at 25 °C [77 °F], 100 kPa) .N check (what is there?)YNLinks to infoboxes
Sodium iodide
(chemical formula
NaI
) is an ionic compound formed from the chemical reaction of the metal sodium and iodine. Under standard conditions, it is a white, water-soluble solid containing a 1:1 mixture of sodium cations (Na + ) and iodide anions (I - ) in a crystal lattice. It is used primarily as a food additive and in organic chemistry. It is produced industrially as a salt formed by the reaction of acid iodides with sodium hydroxide.[11] It is a chaotropic salt.
Uses [edit]
Dietary supplement[edit]
Sodium iodide as well as potassium iodide are commonly used to treat and prevent iodine deficiency. Iodized table salt contains 10 ppm iodide. [eleven]
Organic synthesis[edit]
Monoatomic NaI chains grown inside double carbon nanotubes. [12]
Sodium iodide is used to convert alkyl chlorides to alkyl iodides. This method, the Finkelstein reaction,[13] relies on the insolubility of sodium chloride in acetone, which drives the reaction:[14]
R – Cl + NaI → R – I + NaCl
Nuclear medicine[edit]
Some radioactive sodium iodide salts, including Na125I and Na131I, have radiopharmaceutical uses, such as in the treatment of thyroid cancer and hyperthyroidism or as radiolabeled tracers in imaging (see Isotopes of Iodine > Radioiodine I-123 , I-124, I-125 and I-131 in medicine and biology).
NaI (Tl) scintillators doped with thallium[edit]
Sodium iodide is activated with thallium, NaI (Tl), when exposed to ionizing radiation emits photons (i.e. scintillation) and is used in scintillation detectors, traditionally in nuclear medicine, geophysics, nuclear physics and environmental measurements. NaI (Tl) is the most widely used scintillation material. The crystals are usually coupled to a photomultiplier tube in a sealed assembly because sodium iodide is hygroscopic. Fine tuning of some parameters (for example, radiation resistance, afterglow, transparency) can be achieved by varying the crystal growth conditions. Crystals with higher doping levels are used in X-ray detectors with high spectrometric quality. For this purpose, sodium iodide can be used either as single crystals or as polycrystals. The maximum emission wavelength is 415 nm. [15]
Sodium iodide Na131I (Sodium iodide, 131I)
A solution with a certain radioactivity must be administered intravenously to patients directly.
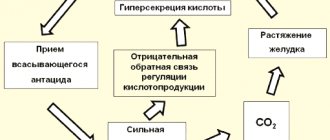
Radioiodine therapy
When treating thyrotoxicosis with diffuse and multinodular toxic goiter, treatment is carried out 3-4 weeks after thyroidectomy or withdrawal of L-thyroxine 20 days before administration of the drug. Patients are transferred to specialized wards equipped with an autonomous ventilation and sewage system connected to special treatment facilities. Patients are removed from the “closed” mode when the gamma radiation power is reduced to that permitted by radiation safety standards (3 µSv/h).
When treating thyrotoxicosis, the amount of the administered drug is selected individually, ranging from 111 to 555 MBq.
The main condition for radioiodine therapy for cancer and metastases of the thyroid gland is thyroidectomy. The duration of treatment is no earlier than 4-6 weeks after surgery. The most optimal form for radioiodine therapy is well-differentiated A-cell thyroid cancer. Radioiodine therapy for metastases of B- and C-cell forms of thyroid cancer is not advisable.
When treating metastases of thyroid cancer, the drug is prescribed orally in the amount of 1850 - 3700 MBq once every 3 months. Before each re-prescription of the drug, studies of the iodine absorption activity of metastases are carried out. To do this, use the method of scintigraphy or radioisotope scanning after administration of 37 - 74 MBq of the drug. The duration of therapy can reach 2 years, and the total dose of 131I is 18.5-25.9 MBq.
There are currently two most common methods for calculating iodine-131 injected activity.
Individual calculation based on thyroid volume, iodine-131 uptake rate during a diagnostic scan 24 hours after dosing, and target activity per gram of tissue (range 0.1 to 0.3 MBq/g) using the formula:
Av = (Az x V) / (C x 10), where
Az—specified activity, MBq/g;
V—volume of the thyroid gland, cm3;
C is the rate of iodine-131 uptake 24 hours after drug administration;
10 is the coefficient.
Purpose of fixed activity of iodine-131:
190 MBq - small glands;
380 MBq - medium-sized glands;
579 MBq - large glands.
Before starting treatment, a preliminary determination of the absorption of iodine-131 by the thyroid gland is necessary, which guarantees the correctness of treatment and eliminates the possibility of error associated with the use of a fixed activity in a patient with a large but poorly absorbing iodine-131 gland.
When using the drug therapeutically, constant monitoring of the state of peripheral blood is a prerequisite.
Radiodiagnosis
Patient preparation. A study of the functional state of the thyroid gland should be carried out no earlier than 4-6 weeks after discontinuation of stable iodine preparations, iodine-containing and iodized foods and multivitamin preparations containing iodine, iodine-containing X-ray contrast agents, fluorine, bromine, thiodothyronine, thyroxine, thyroidine, 6 -methylthiouracil and other similar antithyroid drugs, as well as glucocorticosteroids.
To study the function of the thyroid gland by the amount of iodine-131 accumulation, it is enough to administer 0.037-0.074 MBq of the drug; when scanning the thyroid gland, determining protein-bound iodine and studying the whole body using radiometry - 0.111-0.185 MBq.
Research methods
Functional state of the thyroid gland
can be assessed by:
-the amount of accumulation of iodine-131 in the gland after 2, 4, 24 hours and at a later date after using the drug;
- level of protein-bound iodine in plasma;
-results of whole body radiometry.
The amount of 131I accumulation in the thyroid gland
is a summary indicator of the state of the inorganic and organic phases of iodine metabolism in this organ. The determination is carried out using a radiometer, placing the end of the sensor at a distance of 30 cm from the front surface of the neck. Radiometry of the standard, which is used as 131I in an amount equal to that administered to the patient, is carried out under the same geometric conditions.
The percentage of radionuclide accumulation in the thyroid gland (A) is calculated using the formula:
A = [B-Nф/ C-Nф] x 100%, where:
B—131I content in iron, pulses/min;
C—131I content in the standard, imp/min;
Nf - background, imp/min.
In healthy people, an average of 14% of the administered amount of isotope accumulates in the thyroid gland after 2 hours, 19% after 4 hours, and 27% after 24 hours.
To determine the level of protein-bound iodine
After 48 hours, a blood sample (8-10 ml) is taken from the patient’s antecubital vein. After centrifugation, 4 - 5 ml of blood plasma is transferred into a test tube and the protein is separated by adding a 10% trichloroacetic acid solution three times in a volume equal to the volume of the blood plasma under study, followed by centrifugation at 2000 rpm for 10 minutes. The resulting precipitate is dissolved in a 2 M solution of sodium hydroxide or potassium hydroxide, bringing it to the original volume of blood plasma, and radiometered in a well counter in parallel with the standard. As the latter, a solution of 131I is used, diluted in a ratio of 1:500; the volume of the standard must be equal to the volume of blood plasma taken for radiometry.
The percentage of protein-bound iodine (A) is calculated using the formula:
A = (B - Nph) x 1000 x 100/C x (D - Nph) x 500%/l, where:
B—131I content in iron, pulses/min;
C—volume of blood plasma taken for analysis, ml;
D—131I content in the standard, pulses/min;
Nf - background, imp/min.
The normal level of protein-bound iodine is no more than 0.3% per liter.
Whole body radiometry
allows you to evaluate the peripheral stage of thyroid hormone metabolism and is carried out as follows: 2 hours after taking 1.0 MBq of the drug, during which the patient is asked not to empty the bladder, the first measurement is carried out using a special sensor with a diameter of 10-20 cm in a geometry that provides the necessary accuracy; the results of this first radiometry are taken as 100%; subsequently, radiometry is repeated after 24, 72, 120 and 192 hours; Registration is carried out each time both with shielding of the neck area (thyroid gland) with a lead plate 4-5 cm thick, and without shielding. Based on the results of radiometry, the 131I content in subsequent periods is calculated in the thyroid gland and in the entire body, with the exception of the thyroid gland, as a percentage of the administered amount.
The normal 131I content in the human body (excluding the thyroid gland) after 1 day is 10-25%, after 3 days - 9.7-15%, after 8 days - 2-12%.
The method of radionuclide diagnosis of A-cell cancer and metastases of the thyroid gland includes scanning or scintigraphy of the whole body 24 and 48 hours after intravenous administration of 111-165 MBq of the drug.
Solubility data[edit]
Sodium iodide is highly soluble in some organic solvents, unlike sodium chloride or even sodium bromide:
| Solvent | Solubility of NaI (g NaI/kg solvent at 25 °C) [16] |
| H2O | 1842 |
| Liquid ammonia | 1620 |
| Liquid sulfur dioxide | 150 |
| Methanol | 625–830 |
| Formic acid | 618 |
| Acetonitrile | 249 |
| Acetone | 504 |
| Formamide | 570–850 |
| Acetamide | 323 (41.5 °C) |
| Dimethylformamide | 37–64 |
| Dichloromethane | 0,09 [17] |
Links[edit]
- ^ B s d e e Haynes, pp. 4.86
- Seidell, Atherton (1919). Solubility of inorganic and organic compounds c. 2. D. Van Nostrand Company. paragraph 655.
- Jump up
↑ Haynes, p. 5.171 - Miyata, Takeo (1969). "Exciton structure of NaI and NaBr". Journal of the Physical Society of Japan
.
27
(1): 266. Bibcode: 1969JPSJ ... 27..266M. DOI: 10.1143/JPSJ.27.266. - Guizzetti, G.; Nosenzo, L.; Reguzzoni, E. (1977). "Optical properties and electronic structure of alkali metal halides by thermal reflection." Physical Review
B.
15
(12):5921–5926. Bibcode: 1977PhRvB..15.5921G. DOI: 10.1103/PhysRevB.15.5921. - Jump up
↑ Haynes, p. 4,130 - Jump up
↑ Haynes, p. 10,250 - Davey, Wheeler P. (1923). "Precision measurements of alkali metal halide crystals". Physical Review
.
21
(2): 143–161. Authorization code: 1923PhRv…21..143D. DOI: 10.1103/PhysRev.21.143. - Jump up
↑ Haynes, p. 5.36 - "Sodium iodide 383112". Sigma Aldrich
. - ^ab Lyday, Phyllis A. (2005). "Iodine and iodine compounds". Ullman Encyclopedia of Industrial Chemistry
. Weinheim: Wiley-VCH. pp. 382–390. DOI: 10.1002/14356007.a14_381. - Senga, Ryosuke; Suenaga, Kazu (2015). "Spectroscopy of monatomic electron energy loss of light elements". Nature Communications
.
6
: 7943. Bibcode: 2015NatCo... 6.7943S. DOI: 10.1038/ncomms8943. PMC 4532884. PMID 26228378. - Finkelstein, Hank (1910). "Darstellung organischer Jodide aus den entsprechenden Bromiden und Chloriden". Ber. Dtsch. Chem. Ges.
(German).
43
(2):1528–1532. DOI: 10.1002/cber.19100430257. - Streitwieser, Andrew (1956). "Solvolytic displacement reactions on saturated carbon atoms". Chemical Reviews
.
56
(4):571–752. DOI: 10.1021/cr50010a001. - "Scintillation Materials and Assemblies" (PDF). Crystals of Saint-Gobain. 2021. Retrieved June 21, 2021.
- Burgess, John (1978). Metal ions in solution
. Ellis Horwood Series in Chemical Sciences. New York: Ellis Horwood. ISBN 9780470262931. - De Namor, Angela F. Danil; Traboulssi, Rafik; Salazar, Franz Fernandez; De Acosta, Vilma Dianderas; De Viscardo, Iboni Fernandez; Portugal, Jaime Muñoz (1989). "Transfer and distribution of free energies of 1:1 electrolytes in the solvent system water - dichloromethane at 298.15 K." Journal of the Chemical Society, Proceedings of Faraday 1
.
85
(9):2705–2712. DOI: 10.1039/F19898502705.
Sodium iodide
- General information.
Formula:
NaJ
Sodium iodide NaI.
Colorless transparent crystals. It dissolves well in water and alcohol. There are a number of crystalline hydrates (NaJ.2H2O, NaJ.5H2O, etc.) and polyiodides NaI3 and NaI5. The molar electrical conductivity at infinite dilution at 25°C is 126.9 S.cm2/mol [4].
| -288.06 kJ/mol; | -284.84 kJ/mol; | 98.6 J/mol.K [3]. |
Boiling point: 1300 ºC
Melting point: 651° C
Density: 3.66
Solubility in water, g/100 ml at 20°C: 179.320
- Receipt.
Sodium iodide (sodium iodide) is obtained by reacting I2 or an iodine-air mixture with sodium hydroxide solutions, followed by reduction of the resulting NaIO3 with hydrogen sulfide, hydrogen peroxide, or others, or by reacting sodium hydroxide, soda or sodium sulfate with hydroiodic acid.
- Qualitative analysis.
- Analytical reactions for sodium cation.
1. Reaction with zinc dioxouran(VI) acetate Zn(UO 2 ) 3 (CH 3 COO) 8
with the formation of a yellow crystalline precipitate (pharmacopoeial reaction - GF) or yellow crystals of tetrahedral and octahedral shape, insoluble in acetic acid (MCA). To increase the sensitivity of the reaction, the test mixture should be heated on a glass slide.
NaCl
+ Zn(UO2)3(CH3COO)8 + CH3COOH + 9 H2O
NaZn(UO2)3(CH3COO)9 9 H2O + HCl
Interfering ions: excess K+ ions, heavy metal cations (Hg22+, Hg2+, Sn2+, Sb3+, Bi3+, Fe3+, etc.). The reaction is used as a fractional reaction after removing interfering cations.
2. Coloring the colorless burner flame yellow (YF).
3. Reaction with picric acid to form yellow, needle-shaped sodium picrate crystals emanating from one point (ISS).
Error: Reference source not found
The reaction is used as a fractional reaction only in the absence of interfering ions (K+, NH4+, Ag+).
4. Reaction with potassium hexahydroxostibate(V) K[Sb(OH) 6 ]
with the formation of a white crystalline precipitate, soluble in alkalis.
NaCl
+ K[Sb(OH)6] Na[Sb(OH)6] + KCl
Reaction conditions: a) sufficient concentration of Na+; b) neutral reaction of the solution; c) carrying out the reaction in the cold; d) rubbing a glass rod against the wall of the test tube. Interfering ions: NH4+, Mg2+, etc.
In an acidic environment, the reagent is destroyed with the formation of a white amorphous precipitate of metaantimony acid HSbO3.
K[Sb(OH)6] + HCl KCl + H3SbO4 + 2 H2O
H3SbO4 HSbO3 + H2O
3.2. Analytical reactions for iodide ion.
1. With a group reagent - AgNO3(GF) solution.
Methodology:
to 2 drops of iodide solution acidified with nitric acid, add 1-2 drops of silver nitrate solution, a yellow cheesy precipitate is formed, insoluble in ammonia solution and diluted HNO3.
2. With iron(III) chloride or sodium nitrite in an acidic medium (extraction method) (GF).
2 I—
+ 2 Fe3+ I2 + 2 Fe2+
2 I—
+ 2 NO2- + 4 H+ I2 + 2 NO + 2 H2O
Methodology:
To 2-3 drops of a solution containing iodide ions, add 1-2 drops of dilute sulfuric acid, 0.5 cm3 of chloroform, 2-3 drops of a solution of iron(III) chloride or sodium nitrite, shake vigorously. The chloroform layer turns pinkish-violet. When starch solution is added, a blue color appears.
NOTE: The detection of iodide ions with iron(III) chloride is interfered with by thiocyanate and acetate ions.
Sodium nitrite can be used to open iodide ions using the drop method:
Methodology:
1 drop of solutions of starch, 2 mol/dm3 CH3COOH, iodide and KNO2 is applied successively to the filter paper. A blue spot or ring is observed.
The reaction with sodium nitrite in an acidic environment is specific and makes it possible to detect iodide ions in the presence of chloride, bromide, and acetate ions.
3. With concentrated sulfuric acid (HF).
2 HI
+ H2SO4(conc.) I2 + SO2 + 2 H2O
Methodology:
When 0.1 g of a substance is heated with 1 cm3 of concentrated H2SO4, violet iodine vapor is released.
4. With chloramine B (chlorine water) with the release of molecular iodine.
5. With lead(II) salts to form a yellow precipitate.
2 I
— + Pb2+ PbI2
- Quantitative analysis.
3.1. Argentometry.
Determination of the mass fraction of sodium iodide in a solution using the Faience argentometric method (direct titration option)
The determination is based on the precipitation of iodide ions with a solution of silver nitrate in acetic acid in the presence of the adsorption indicator eosin.
1. K I
+ AgNO 3 Ag I + KNO 3
2. {(Ag I ) n
n I - (nx) K} x-
xK +
3. [(AgI) n ]
4. {(AgI) n
nAg + (nx) NO 3 - } x+
x NO 3
-
at the end point of the titration:
5. {(Ag I ) n
nAg + (nx) Jnd - } x+
x NO 3 -
M (KI) = 166.01 g/mol
M (NaI) = 149.89 g/mol
Methodology:
The exact volume of potassium iodide solution (individual assignment) is placed in a titration flask, 2-3 drops of eosin indicator solution, 10 drops of diluted (30%) acetic acid are added and titrated with 0.05 M silver nitrate solution until the precipitate turns bright pink. .
- Application.
Sodium iodide (Sodium iodide) is used • for the production of single crystals, • in the pharmaceutical industry and medicine, • in veterinary medicine and agriculture, • as a scintillator, an electrolyte component in chemical current sources and electrochemical converters, etc.
- Bibliography.
- Lurie Yu.Yu. Handbook of Analytical Chemistry. Moscow, 1972;
- Methodological instructions “Instrumental methods of analysis”, Perm, 2004;
- Methodological instructions “Qualitative chemical analysis”, Perm, 2003;
- Methodological instructions “Quantitative chemical analysis”, Perm, 2004;
- Rabinovich V.A., Khavin Z.Ya. Brief chemical reference book, Leningrad, 1991;
- "Great Soviet Encyclopedia";
- https://ru.wikipedia.org/.