Staloral Mite allergen, 3 pcs., 10 IR/ml, sublingual drops (set), Initial course
The effectiveness of ASIT is higher in cases where treatment is started in the early stages of the disease.
Dosage and treatment regimen The dosage of the drug and the regimen for its use are the same for all ages, but can be changed depending on the individual reactivity of the patient. The attending physician adjusts the dosage and treatment regimen in accordance with possible symptomatic changes in the patient and individual response to the drug. Treatment consists of two stages: initial and maintenance therapy.
1. Initial therapy begins with a daily dose of the drug at a concentration of 10 IR/ml (blue bottle cap) with one click on the dispenser and gradually increases the daily dosage to 10 clicks. One press of the dispenser is about 0.1 ml of the drug. Next, they proceed to daily administration of the drug at a concentration of 300 IR/ml (purple bottle cap), starting with one press and gradually increasing the number of presses to the optimal (well tolerated by the patient). The first stage can last 9 - 21 days. During this period, the maximum dosage is reached, individual for each patient (from 4 to 8 presses daily of the drug with a concentration of 300 IR/ml), after which they proceed to the second stage.
2. Maintenance therapy with a constant dose using a vial of concentration 300 IR/ml. The optimal dose achieved in the first stage of initial therapy continues to be taken in the second stage of maintenance therapy. Recommended dosage regimen: from 4 to 8 presses on the dispenser daily or 8 presses 3 times a week. Duration of treatment Maintenance therapy is recommended for 3-5 years. If improvement has not occurred after the first year of treatment, the advisability of ASIT should be reconsidered. Directions for use: Before taking the drug, make sure that:
- the expiration date has not expired;
- a bottle of the required concentration is used.
It is recommended to take the drug in the morning before breakfast. The drug should be dropped directly under the tongue and held for 2 minutes, then swallowed. Children are recommended to use the drug with the help of adults. To ensure the safety and integrity of the drug, the bottles are hermetically sealed with plastic caps and rolled with aluminum caps.
Break in taking the drug If you miss taking the drug for a long time, you should consult your doctor. If you miss taking the drug for less than one week, it is recommended to continue treatment without changes. If the gap in taking the drug was more than one week at the initial stage or during maintenance therapy, it is recommended to carry out treatment again with one click on the dispenser, using the same concentration of the drug (as before the break), and then increase the number of clicks, according to the scheme of the initial stage of therapy to the optimal well-tolerated dose.
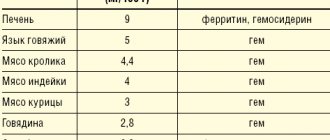
Seasonal allergies (hay fever)
- The allergen is taken once a day - in the morning or evening before meals and brushing teeth
- The appropriate amount of the allergen “Staloral Birch” or “Staloral Mites”
using a dispenser (
count PRESSES! not drops
), it
must be held in the mouth for about 2 minutes
and swallowed.
Store the allergen in its original packaging in the refrigerator (t +4-+8°C). Wash your hands. Do not drink or eat anything allergen for 40 minutes
, wait these 40 minutes before brushing your teeth - Allergen "Oralair"
does not require refrigeration - For the entire period of allergen treatment and dusting, follow a diet excluding your cross-allergens (see below)
- During treatment, a call to the allergist is required when switching to a bottle with a purple cap and a scheduled examination once every 2-3 months (in April - for those who are allergic to tree pollen; in June - for those who are allergic to meadow grass pollen =cereals; in August-September and March-April – for allergies to house dust mites). At least once a year, do a General blood test with leukemia and ESR, a General urine test, a feces test for worm eggs and a scraping for enterobiasis.
- When opening a new bottle of Staloral, always use a new dispenser and make the first 3-5 presses into the sink (it is necessary to “drive” the air out of the dispenser for accurate dosing of the allergen).
- The interval between doses of the allergen with different concentrations is unacceptable (i.e. between the blue and purple bottles of “Staloral”).
- An opened bottle of “Staloral” can be used for no longer than 2 months. Therefore: 1) if for one reason or another you interrupted treatment for more than 2 weeks, contact your allergist before resuming ASIT; 2) the blue bottle should be thrown away 2 months after opening; 3) we recommend taking the purple bottle you started “to the last drop,” even if it seems to you that the dusting of trees in your region has already stopped or you are going on vacation to another region in May and the first half of June.
- For acute diseases: a break from ASIT is necessary - no more than 8-10 days - and consultation with an allergist before resuming taking the allergen
- If vaccination or tuberculin diagnostics is necessary: stop allergen treatment a week before vaccination; the break after vaccination should be 2 weeks (inactivated vaccines), 4 weeks (live vaccines, for example, against measles, mumps, rubella, chickenpox, oral polio vaccine), 8-12 weeks (after BCG). We recommend scheduling vaccinations and tuba. samples, diaskin test for the season when you do not take the allergen and dusting has not yet begun (for example, September-October-November-the first half of December)
- If undesirable effects occur: itching in the mouth/ears, nasal congestion, sore throat, coughing, sneezing, rash, etc. for longer than 20 minutes, rinse your mouth with water, take an antihistamine (any of the following in a single age dose) and consult an allergist
- from 12 years: Rupafin or Allegra-180 (Fexadin-180) or Erius (Dezal, Lordestin, Desloratadine-TEVA,
Ezlor) or Nixar, Kestin-BR - sublingually 1 tablet * 1 r / day 1 tablet * 1 r / day
- from 6 years: - Erius (Dezal) syrup - 5 ml*1r/denili
— Xyzal (Suprastinex, Glencet) or Zyrtec (Zodak, Cetrin, Letizen) 1 tablet *1 ruble/day
__________________________________________
If symptoms recur for several days in a row, you can take the indicated drug the evening before for at least 7-15 days continuously: this alleviates the unpleasant manifestations of itching and will allow you to continue therapy.
DIET REMINDER during ASIT and dusting (cross plant allergens)
| I am allergic to pollen... | Foods and drinks that may contribute to adverse events and should be avoided | Medicinal plants and components of cosmetics |
| TREES (birch, alder, willow, hornbeam, hazel, oak, elm, ash, maple, etc.) Dusting season*: 5/IV-20/VI ASIT | Honey and bee products, birch sap, raw apples, pears, kiwis, cherries, cherries, plums, peaches, apricots, nectarines, quinces, persimmons, jack fruit (including juices and smoothies made from them), sometimes black olives, bananas tree nuts (hazelnuts, walnuts, almonds, pecans, pistachios, macadamia, cashews, pine), sometimes peanuts, incl. in the composition of chocolates, chocolate spread, peanut butter, cognac, cider, Calvados, raw carrots and potatoes, herbs and spices (celery, dill, parsley, curry, anise, cumin, onion), peas, tomatoes, cucumbers. | Birch leaf, birch buds, birch tar, alder cones, oak bark, buckthorn bark, pine buds and others. Carefully! – tomato seedlings and cosmetics with herbal ingredients (shampoos, shower gels, creams, toothpaste, etc.) Herbal medicine, aromatherapy, essential oils are excluded! |
| CEREAL (MEADOW) HERBS: timothy, fescue, cocksfoot, bluegrass, foxtail, ryegrass, oats, wheat, barley, rye, corn, etc. Dusting season*: 10/VI-10/VIII ASIT drug "Oralair" | Honey and bee products, chak-chak. Muesli and muesli bars, baked goods (baked goods, pancakes, etc.) or products containing flour, bran (for example, breaded, battered); beer, kvass, vodka, mead, coffee substitutes and even coffee itself corn, peanuts, legumes, strawberries, wild strawberries, citrus fruits, sorrel | All cereal herbs for example, oats, corn silk, bran, wheat germ, oat milk, etc. Herbal medicine, aromatherapy, essential oils are excluded! |
During the flowering (dusting) period of “dangerous plants” you should:
- refrain from traveling outside the city and walking in forested areas
- keep the windows tightly closed, and if necessary, put a gauze net on the window, constantly moistening it. Option - use an air conditioner and an air purifier (and in the car as well)
- when going outside: wear safety glasses, hats and use “ Nazaval”
"(any age, pregnant, lactating - without restrictions), "
Prevalin
" (from 6 years old - children's, from 12 years old - regular), intranasal HEPA filters (from 4 years old, size S; from 16-18l for men, size L), "
AquaMaris-Ectoin
”, and upon return, rinse the mucous membranes of the nose and eyes, take a shower and wash your hair.
- keep a diary of symptoms, also noting medications taken in it
- it is best to go to another climate zone, where there is no “dangerous” plant or it does not bloom. If you haven’t left, start pre-seasonal treatment with an allergist
- Postpone scheduled preventive vaccinations, tuberculin tests (Mantoux, Pirke) and Diaskin test, surgical interventions, extraction (removal) and prosthetics of teeth, X-ray contrast and endoscopic types of medical examination to another time of the year.
Look for Pollen Monitoring information in the relevant region on the Internet (www.new.paracels.net - Tyumen, www. allergotop.com - Moscow and Moscow Region, www.microvita.ru - Central Europe, www.polleninfo.org - European Union countries) .
Participate in the PollenClub project yourself (mobile application and website, the Archive contains information on past dusting seasons, linked to a world map).
* DUST CALENDAR for different regions of Russia - www.new.paracels.net - in the section “Patients: Seasonal allergies”
At the end of the ASIT season
Give your Procedure Sheet and Symptom Diary to your allergist - analyze them together and plan the next season of therapy.
Remember: TREATMENT WITH THE ASIT METHOD TAKES 4-5 YEARS, and the patient bears equal responsibility with the doctor for the success and safety of allergen treatment!
CONSULT YOUR DOCTOR WITH ANY QUESTIONS!
Staloral birch pollen allergen maintenance course
INDICATIONS FOR USE Allergen specific immunotherapy (ASIT) for patients with allergic reaction type 1 (IgE mediated), suffering from rhinitis, conjunctivitis, mild or moderate form of seasonal bronchial asthma, and hypersensitivity to birch pollen. Immunotherapy can be administered to adults and children from 5 years of age.
CONTRAINDICATIONS
- Hypersensitivity to one of the excipients (see list of excipients);
- Autoimmune diseases, immune complex diseases, immunodeficiencies;
- Malignant neoplasms;
- Uncontrolled or severe bronchial asthma (forced expiratory volume <70%);
- Therapy with beta-blockers (including local therapy in ophthalmology);
- Severe inflammatory diseases of the oral mucosa, for example, erosive-ulcerative form of lichen planus, mycoses.
METHOD OF APPLICATION AND DOSAGE The effectiveness of ASIT is higher in cases where treatment is started in the early stages of the disease. Dosage and treatment regimen The dosage of the drug and the regimen for its use are the same for all ages, but can be changed depending on the individual reactivity of the patient. The attending physician adjusts the dosage and treatment regimen in accordance with possible symptomatic changes in the patient and individual response to the drug. It is advisable to start treatment no later than 2-3 months before the expected flowering season and continue throughout the entire flowering period. Treatment consists of two stages: initial and maintenance therapy.
1. Initial therapy begins with a daily dose of the drug at a concentration of 10 IR/ml (blue bottle cap) with one click on the dispenser and gradually increases the daily dosage to 10 clicks. One press of the dispenser is about 0.1 ml of the drug. Next, they proceed to daily administration of the drug at a concentration of 300 IR/ml (purple bottle cap), starting with one press and gradually increasing the number of presses to the optimal (well tolerated by the patient). The first stage can last 9 - 21 days. During this period, the maximum dosage is reached, individual for each patient (from 4 to 8 presses daily of the drug with a concentration of 300 IR/ml), after which they proceed to the second stage.
Recommended scheme for the initial ASIT course:
| Bottle 10 TS/ml (blue cap) | Bottle 300 TS/ml (purple cap) | ||
| Day | Number of presses on the dispenser | Day | Number of presses on the dispenser |
| 1 | 1 | 7 | 1 |
| 2 | 2 | 8 | 2 |
| 3 | 4 | 9 | 4 |
| 4 | 6 | 10 | 6 |
| 5 | 8 | 11 | 8 |
| 6 | 10 | ||
2. Maintenance therapy with a constant dose using a vial of concentration 300 IR/ml. The optimal dose achieved in the first stage of initial therapy continues to be taken in the second stage of maintenance therapy. Recommended dosage regimen: from 4 to 8 presses on the dispenser daily or 8 presses 3 times a week.
Duration of treatment Allergen specific immunotherapy is recommended to be carried out in the above two-stage courses (2-3 months before the expected flowering season until the end of the season) for 3-5 years. If, after treatment, improvement does not occur during the first flowering season, the feasibility of ASIT should be reconsidered.
Directions for use: Before taking the drug, make sure that:
- the expiration date has not expired;
- a bottle of the required concentration is used.
It is recommended to take the drug in the morning before breakfast. The drug should be dropped directly under the tongue and held for 2 minutes, then swallowed. Children are recommended to use the drug with the help of adults.
To ensure the safety and integrity of the drug, the bottles are hermetically sealed with plastic caps and rolled with aluminum caps. For first use, open the bottle as follows:
1/ Tear off the colored plastic cap from the bottle.
2/ Pull the metal ring to remove the aluminum cap completely.
3/ Remove the rubber plug.
4/ Remove the dispenser from the plastic packaging. Holding the bottle firmly with one hand, with the other hand, pressing firmly on the top flat surface of the dispenser, snap it onto the bottle.
5/ Remove the orange protective ring.
6/ Press the dispenser firmly 5 times over the sink. After five clicks, the dispenser dispenses the required amount of the drug.
7/ Place the dispenser tip in your mouth under your tongue. Press the dispenser firmly as many times as prescribed by your doctor to obtain the required amount of the drug. Hold the liquid under your tongue for 2 minutes.
8/ After use, wipe the pipette tip and put on the protective ring.
For subsequent use, remove the protective ring and follow steps 7 and 8.
Break in taking the drug If you miss taking the drug for a long time, you should consult your doctor. If you miss taking the drug for less than one week, it is recommended to continue treatment without changes. If the gap in taking the drug was more than one week at the initial stage or during maintenance therapy, it is recommended to carry out treatment again with one click on the dispenser, using the same concentration of the drug (as before the break), and then increase the number of clicks, according to the scheme of the initial stage of therapy to the optimal well-tolerated dose.
SIDE EFFECTS Carrying out ASIT can cause adverse reactions, both local and general. The dosage and treatment regimen may be revised by the attending physician in case of an individual reaction or changes in the general condition of the patient. Local reactions:
- oral: itching in the mouth, swelling, discomfort in the mouth and throat, disruption of the salivary glands (increased salivation or dry mouth);
- gastroenterological reactions: abdominal pain, nausea, diarrhea.
Usually these symptoms go away quickly, and there is no need to change the dosage or treatment regimen. If symptoms occur frequently, the possibility of continuing therapy should be reconsidered. General reactions are rare:
- rhinitis, conjunctivitis, asthma, urticaria require symptomatic treatment with H1-antagonists, beta-2 mimetics or corticosteroids (orally). The physician should reconsider the dosage and treatment regimen or the possibility of continuing ASIT.
- in extremely rare cases, generalized urticaria, angioedema, laryngeal edema, severe asthma, anaphylactic shock are possible, which requires the abolition of ASIT.
Rare side effects not related to Ig-E mediator reactions:
- asthenia, headache;
- exacerbation of preclinical atopic eczema;
- delayed reactions of the serum sickness type with arthralgia, myalgia, urticaria, nausea, adenopathy, fever, which requires the abolition of ASIT.
All side effects should be reported to your doctor.
OVERDOSE If the prescribed dose is exceeded, the risk of side effects increases, which requires symptomatic treatment.
DRUG INTERACTIONS Do not use simultaneously with beta-blockers. Possible simultaneous use with symptomatic antiallergic drugs (H1-antihistamines, beta-2 mimetics, corticoids, mast cell degranulation inhibitors) for better tolerability of ASIT.
PREGNANCY AND BREASTFEEDING Pregnancy You should not start ASIT during pregnancy. If pregnancy occurs during the first stage of treatment, therapy should be discontinued. If pregnancy occurs during the period of maintenance therapy, the doctor should assess the possible benefits of ASIT based on the general condition of the patient. No side effects have been reported with the use of ASIT in pregnant women. Breastfeeding It is not recommended to start a course of ASIT during breastfeeding. If a woman continues to perform ASIT during lactation, no adverse symptoms or reactions in children are expected. There are no clinical data on the use of the drug during lactation.
WARNINGS AND PRECAUTIONS If necessary, before starting ASIT, allergy symptoms should be stabilized with appropriate therapy. Patients undergoing ASIT should always carry medications to relieve allergy symptoms, such as corticosteroids, sympathomimetic drugs and antihistamines. You should immediately consult a doctor if you experience severe itching of the palms, arms, soles of your feet, urticaria, swelling of the lips, larynx, accompanied by difficulty swallowing, breathing, or change in voice. In these cases, your doctor may recommend taking epinephrine. In patients taking tricyclic antidepressants, monoamine oxidase inhibitors, the risk of side effects of epinephrine increases, including death. This circumstance should be taken into account when prescribing ASIT. In case of inflammatory processes in the oral cavity (mycoses, aphthae, gum damage, tooth extraction/loss or surgery), therapy should be interrupted until the inflammation is completely cured (at least for 7 days). During the ASIT course, vaccination may be carried out after consultation with a doctor. For patients, especially children, on a diet with reduced salt intake, it should be taken into account that the drug contains sodium chloride (one press of the dispenser is about 0.1 ml of the drug containing 5.9 mg of sodium chloride). When traveling, make sure that the bottle is in an upright position. The bottle should be in a box with a protective ring on the dispenser. The bottle should be placed in the refrigerator as soon as possible.
RELEASE FORM 10 ml of allergen containing 10 IR/ml and 300 IR/ml in glass bottles with a capacity of 14 ml closed with rubber stoppers, rolled with aluminum caps with plastic lids in blue (10 IR/ml) and violet (300 IR/ml).
STORAGE AND TRANSPORTATION CONDITIONS Store and transport at temperatures from 2 to 8 °C. Keep out of the reach of children.
SHELF LIFE 36 months. Do not use after expiration date.
special instructions
ASIT should not be started during pregnancy.
If pregnancy occurs during the first stage of treatment, therapy should be discontinued. If pregnancy occurs during the period of maintenance therapy, the doctor should assess the possible benefits of ASIT based on the general condition of the patient.
No side effects have been reported with the use of ASIT in pregnant women.
It is not recommended to start a course of ASIT during breastfeeding.
If a woman continues to perform ASIT during lactation, no adverse symptoms or reactions in children are expected.
There are no clinical data on the use of the drug during lactation.
For patients, especially children, on a diet with reduced salt intake, it should be taken into account that the drug contains sodium chloride (one press of the dispenser is about 0.1 ml of the drug containing 5.9 mg of sodium chloride).
When traveling, make sure that the bottle is in an upright position. The bottle should be in a box with a protective ring on the dispenser. The bottle should be placed in the refrigerator as soon as possible.