Infusion (“drip”) is a method of slowly introducing a substance in the form of a solution into a person’s vascular bed. Doctors of almost all specializations use infusion therapy in their practice and cannot imagine modern medicine without it.
Infusion is carried out for various purposes:
- Administration of medications (painkillers, antibiotics, etc.).
To comply with the conditions of therapy with some drugs, it is important to maintain a certain concentration of the substance in the blood for a given time. A decrease or increase in concentration reduces the effect of therapy to a minimum, and may have the opposite effect.
- Delivery of nutrients.
To maintain the vital functions of the body, for example, in a patient with difficulty in self-feeding, infusions are administered to help increase the concentration of proteins, hemoglobin, etc. and normalization of metabolic processes.
- Replenishment of lost fluid (extracellular or intracellular);
- Infusion of blood products (correction of blood volume in the body).
Types of infusions
By localization of administration:
Intra-arterial. For intra-arterial administration, a special valve is used;
Intravenous. The substance will enter the venous bed due to its own gravity. To do this, the vessel with the solution is placed at a level more than 10 centimeters above the patient’s position, using a special tripod.
According to the method of administration, they are distinguished:
Jet. Characterized by minimal dilution of the drug. The jet injection includes bolus administration, due to which the required concentration of the drug in the blood is achieved quickly enough. With slow administration, it takes a long time to achieve the optimal concentration; it is also carried out using infusion pumps (a device for intravenous infusion that resembles a pump in its mode of action). For both methods of administration, syringes are used;
Drip. For drip infusions, a solution of the administered substance is used. This achieves minimal impact on the walls of arteries and veins, and adjusts the volume of infusion.
Video
More photos Author(s):
E.A.
Lezhneva, senior doctor of the department of anesthesia, resuscitation and intensive care of the Bely Klyk clinic Magazine:
No. 6-2017
Key words
: infusion at a constant rate, IPS, calculation formula
Key words:
constant rate infusion, CRI, formula for CRIAbstract
annotation
The article describes methods for calculating drugs for infusion at a constant rate.
Summary
Ways of calculating drugs for CRI are described in this article.
Introduction
Increasingly, there are recommendations for the administration of certain drugs intravenously in constant rate infusion (CI). And if quite recently this was a “headache” for resuscitators calculating doses of dopamine and norepinephrine, now the skill of calculating IPS is becoming necessary for the daily practice of any doctor. Indeed, in addition to vasopressors and cardiotonics, there are other drugs that are often more convenient and better administered in the form of IPS: anesthetics, analgesics, muscle relaxants, prokinetics, diuretics, and sometimes antibiotics.
How to use
Pharmacokinetically, IPA is justified for drugs whose small doses have a short half-life (for example, lidocaine), or for substances with a relatively small volume of distribution, that is, only this method of administration allows maintaining a stable plasma concentration of the active substance.
Unfortunately, IPS is only possible using a syringe pump (infusion pump). In our practice, we use special “infusion guides” - convenient tubes connecting the syringe and the intravenous catheter. It is technically possible to use a cut-off part of a conventional gravity infusion system, but this is incorrect, since the sterility of the system is compromised at the time of its collection. In addition, to connect several infusion lines, it is convenient to use a special three-way tap and T-port.
Special attention should be paid to the choice of carrier solution, i.e. solution with which we will dilute the medicine. For example, dopamine and dobutamine are incompatible with alkaline solutions, i.e. The ideal carrier for them would be isotonic sodium chloride solution. Trisol, on the contrary, will not work.
Let's look at the calculation technique using an example. The data required for this is: the patient’s body weight, the desired dose of the drug per unit of time, the desired rate of administration of the finished solution, the volume of the syringe that we are going to use, the end of the solution. Our goal is to find out the volume of the “mother liquor”, i.e. solution from the ampoule, which must be added to the syringe with the carrier solution.
Example
· Weight 7.5 kg.
· Lidocaine 40 mcg/kg/min (dose of the drug).
· Administration rate 4 ml/h (the infusion rate of the carrier solution set on the infusion pump is selected arbitrarily, depending on the patient’s need for fluid. If we want to immediately administer the required volume of infusion along with the drug, then we can choose a higher rate, if this a patient with pulmonary edema who does not need excess fluid, then you can set the minimum speed (for example, 1 ml/h), that is, you can choose any speed that is convenient or necessary for us.
· 20 ml syringe (if necessary, you can use a 10 ml or 50 ml syringe if the infusion pump allows).
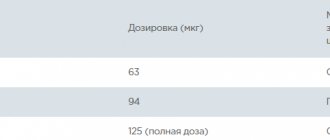
· Lidocaine 2%. 1 ml of 2% solution contains 20 mg of active substance. 1 mg = 1000 mcg, i.e. 1 ml of 2% lidocaine solution contains 20,000 mcg of active substance.
1% solution = 10 mg/ml
1 g = 1000 mg = 1000,000 mcg = 1000,000,000 ng
We use the following calculation algorithm:
1. Multiply the dose by the animal’s body weight by 60 (if the dose is indicated for 1 minute and not for 1 hour).
2. Multiply by the volume of the syringe in ml.
3. Divide by the concentration of the mother solution. IMPORTANT! The weight units (mcg or mg) must be the same in which the dose was calculated!
4. Divide by the rate of introduction of the carrier solution.
Total: you need to take 4.5 ml of a 2% lidocaine solution, bring it to 20.0 ml with a carrier solution, for example, isotonic sodium chloride or Ringer's solution, and administer intravenously at a rate of 4 ml/hour.
This formula combines several step-by-step calculations.
We want to administer lidocaine to a 7.5 kg dog at a rate of 40 mcg/kg/min.
This means that for 1 hour of infusion, such a dog will need 7.5 kg × 40 mcg × 60 min = 18,000 mcg.
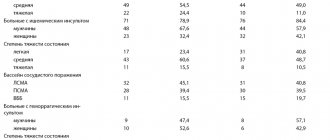
If we want to deliver the solution at a rate of 4 ml/h and use a 20 ml syringe, then we need to draw up the lidocaine solution for 5 hours (20 ml: 4 ml/h = 5 hours, a 20 ml syringe at this rate will be enough for 5 hours).
If 18,000 mcg of lidocaine was needed for 1 hour, then 18,000 mcg × 5 = 90,000 mcg = 90 mg of lidocaine was needed for 5 hours.
We know that 1 ml of 2% lidocaine contains 20 mg of solution; to find out how much you need to take in ml to get 90 mg, you can make a proportion:
1 ml – 20 mg
X ml – 90 mg.
Remembering the rules for calculating proportions from school, we can easily calculate:
X = 90 × 1: 20 = 4.5 ml of 2% lidocaine must be diluted to 20 ml and delivered at a rate of 4 ml/h.
Thus, you can use the formula given above or calculate according to the plan.
1. How much drug does the patient need for 1 hour (weight in kg × dose in mcg/kg × 60 (if the rate is mcg/kg/min, if the rate is mcg/kg/hour, then there is no need to multiply by 60)).
2. Decide how many hours we fill the syringe.
3. Multiply the amount obtained in step 1 by the number of hours - we get the dose of the drug in mcg required for this syringe.
4. We calculate how much it will be in ml, depending on the concentration of the solution in the ampoule.
Important! When using an injection rate that is a multiple of the drug dose, there is no need to repeat the calculation to change the dose; it is enough to change the perfusor speed multiple times. For example, we administer lidocaine at a dose of 40 mcg/kg/min at a rate of 4 ml/h; to change the dose to 25 mcg/kg/min, it is necessary to reduce the speed of the syringe pump to 2.5 ml/h, etc.
The most common errors in calculations are multiplication by a factor of 60 in cases where the dose is indicated for 1 hour (for example, for dexmedetomidine or medetomidine) or for a day (for example, for cerucal), and the use of different weight units for the dose of the drug and the stock solution.
Even after a little training, you can easily calculate the IPA with one equation on a calculator using the formula.
Example 1: Dog 23 kg, dopamine 5 mcg/kg/min, dopamine 4% in ampoule, injection rate 5 ml/h, syringe 50 ml. After some time, it was decided to change the dose to 7 mcg/kg/h. Your actions?
Solution: 23 kg × 5 µg/kg/min × 60 min × 50 ml syringe / 40,000 µg/ml / 5 ml/h = 1.725 ml.
Answer: ≈ 1.7 ml of 4% dopamine solution must be diluted to 50.0 ml and administered at a rate of 5 ml/hour. To increase the dose, you can increase the rate of administration to 7 ml/hour.
Example 2: Cat 5 kg, dexmedetomidine 0.5 μg/kg/h, dexmedetomidine 0.01%, injection rate 10 ml/h, syringe 50 ml.
Solution: 5 kg × 0.5 μg/kg/h × 50 ml syringe / 100 μg/ml / 10 ml/h = 0.125 ml.
Answer: ≈ 0.13 ml of 0.01% dexmedetomidine solution must be diluted to 50.0 ml and administered at a rate of 10 ml/hour.
Example 3: Dog 8 kg, cerucal 2 mg/kg/day, cerucal 5 mg/ml, speed 4 ml/h, syringe 20 ml.
Solution: The dose of the drug is indicated per day, but our task remains unchanged - we need to find out the amount of stock solution to add to the IPA syringe, therefore:
8 kg × 2 mg/kg/day * 20 ml syringe / 24 hours / 5 mg/kg / 4 ml/hour = 0.667 ml.
Answer: ≈0.67 ml of cerucal should be diluted to 20.0 ml and administered at a rate of 4 ml/hour.
Example 4: Dog 9 kg, dobutamine 5 mcg/kg/min, injection rate 2.5 ml/h, dobutamine lyophilized powder, 250 mg vial, 20 ml syringe. After some time, it was decided to change the dose to 7 mcg/kg/min. Your actions?
Solution: Since dobutamine is lyophilized, we first need to prepare a “master” solution, for this we need to dilute it, for example, with 20 ml of 0.9% sodium chloride solution. Thus, we will obtain a stock solution with a concentration of 12.5 mg/ml (we dilute 250 mg in 20 ml, which means 250 / 20 = 12.5 mg/ml = 12,500 μg/ml).
Next, according to the usual formula: 9 kg × 5 mcg/kg/min × 60 min × 20 ml syringe / 12,500 mcg/ml / 2.5 ml/h = 1.728 ml.
Answer: ≈ 1.7 ml of the prepared dobutamine solution is diluted to 20.0 ml and administered at a rate of 2.5 ml/hour. To increase the dose to 7 mcg/kg/min, it is necessary to change the injection rate to 3.5 Back to section
How is the infusion performed?
Infusion can only be performed by medical personnel who have knowledge of the procedure algorithm and practical skills. For infusions, only disposable intravenous drip systems are used, consisting of a dropper with tubes, special needles, and an air duct.
The systems are sterile, the puncture site is treated with an antiseptic. The patient is positioned on the couch, the tube and needle are fixed on his arm. The healthcare professional checks that the venipuncture was performed successfully and adjusts the infusion rate according to the doctor's instructions. After injecting the required volume of liquid, the needle is removed and the system is disassembled.
Currently, the Medicenter has all the capabilities to carry out infusions not only on an outpatient , but also at home , and all nursing staff have the skills to carry out the procedure.
The influence of injection speed, syringe size and model on the initial period of infusion through a perfuser
S.B. Neff*y, T.A. Neffy, S. Gerber*, M.M. Weiss*
*University Children's Hospital Zurich, Department of Anesthesiology; yUniversity Hospital of Zurich, Department of Anesthesiology, Zurich, Switzerland
Accepted for publication November 10, 2006 EJA 4151 First published electronically January 30, 2007
Summary
Relevance and goals:
When carrying out infusion through a perfuser, there is a significant delay in the flow of solution at the initial stage, especially at low rates of administration, which are widely used in pediatric anesthesia and intensive care. This delay occurs due to the slow interaction of parts in the perfuser transmission mechanism and the resistance of the syringe parts. The purpose of this study was to determine the effect of injection rate, syringe size and model on fluid flow during the initial period of infusion.
Methodology:
For different infusion rates (0.1, 0.5, 1 ml/h), different syringe sizes (10-, 20-, 30-, 50-ml) and different brands (BD and Codan), the delay time from the start of infusion to establishing a constant flow. In each case, 4 measurements were carried out using 2 identical Alaris Asena syringe pumps (8 experiments in total). Statistical processing consisted of a 2-way ANOVA test followed by Bonferroni testing; P < 0.05 was considered significant. Results: Ready time ranged from 3.6 + 0.9 min (BD 10 ml syringe, 1.0 ml h) to 74.5 + 26.6 min (BD 50 ml syringe, 0.1 ml h). Overall, ready times were significantly longer with lower flow rates (0.1 mL h and 1 mL h; P, 0.0001), larger syringe sizes (50 mL and 10 mL; P, 0.01), and with BD versus Codan syringes. (P, 0.01). Conclusion: The fastest possible infusion rate, smaller syringe size, and reduced-resistance syringe pistons should be used to avoid significant initial lag when injecting the solution.
Keywords:
equipment and resources; perfusors; syringes.
Introduction
The perfusor is an indispensable tool in anesthesiology and intensive care. It is used to administer concentrated solutions of fast-acting substances, for example, catecholamines or inotropic substances. Continuous intravenous (IV) drug administration in emergency settings requires an accurate and reliable device [1–5]. In particular, neonates and children are highly susceptible to any inaccuracies in the infusion of concentrated solutions, therefore, for example, catecholamines are administered to them at a low rate to avoid volume overload [5-9]. Such irregularity is especially critical in severely ill patients dependent on vasoactive and inotropic drugs used at low rates; this often results in an interaction between changes in hydrostatic pressure and the syringe pump mechanisms. [10-16] . These inconveniences with high-tech perfusers can be avoided by not positioning the perfuser vertically and by not using low-stretch infusion lines [17].
A persistent problem with infusion pumps is significant start-up delay at low infusion rates [18] . At 1.0 ml/h, the delay is 57.2 minutes before the first entry of fluid and 76.2 minutes before a constant flow is established [18]. The delay is mainly due to the mechanical interaction of the syringe and the pump, friction and internal resistance of the perfuser parts [1,8,18].
Although mechanical interference can be counteracted by standard initial procedures (bolus administration) before connection to the patient, systemic factors cannot be eliminated and result in a significant delay in starting the infusion. Therefore, efforts should be directed toward reducing syringe resistance, which will reduce delay, especially at low speeds.
The purpose of the study was to examine the effect of syringe size and design on infusion initiation and flow at twice the infusion rate, as typically occurs in children and neonates.
Methods
Perfusion system
Fluid delivery in this study was determined using a perfusion system consisting of an AsenaTM GH perfuser (Alaris Medical Systems, Hampshire, UK), syringes of various sizes from two brands (10-, 20-, 30- and 50-ml; BD Plastipack, Becton Dickinson, Meylan Cedex, France and Codan; CODAN Medical, ApS, Rodby, Denmark) and 2 m infusion lines (Clinico Medical GmbH, Bad Mersfeld, Germany) connected via a 3-way stopcock to a 0.50 m infusion line. The Asena perfuser was selected based on previous experiments where the device was shown to be superior to similar devices (data not shown). In a previous study, Codan syringes performed better compared to 4 tested samples [12]; BD syringe is another leading brand in Europe. In addition, only these brands have 4 different sizes compatible with the Alaris Asena perfuser at the time of the study. For each experiment, the above infusion system (syringe and infusion line) was filled with distilled water, the remaining air was removed and the syringe was installed in the perfuser. Before connecting the distal end of the infusion line to a single-lumen 22 G central catheter (CVC) (Certofix Mono Paed S 110; B. Braun, Melsungen, Germany), the system was pre-primed with a 1 ml bolus. To simulate a central venous pressure (CVP) of 10 mmHg, the end of the catheter was placed at a depth of 13 cm in a container filled with sterile distilled water. To prevent evaporation of the liquid, the surface was covered with a thin layer of oil. The perfuser was installed in such a way that the outlet of the syringe was at the level of the end of the catheter. Based on the relative mass of water 999.83 kg/m2 at a temperature of 228C and a pressure of 1 atm, the flow of fluid from the catheter into the control container was estimated gravimetrically using electronic balancing (sensitivity 0.0001 g; AG 204-Delta - Range s, Mettler Toledo, Schwerzenbach, Switzerland) and was expressed in ml/hour [19]. The output balance data was recorded every 10 s using the MCPS V 2.6-CAD program (Software GmbH, Moenchengladbach, Germany). The room temperature was maintained at 22-23°C.
Measurements
All experiments were performed with sterile disposable 10-, 20-, 30-, and 50-mL syringes from two manufacturers, BD and Codan. After installing the appropriate syringe into the perfuser, initially filling the system, and connecting the system to the CVC, infusion was started at each rate tested (0.1, 0.5, and 1.0 ml/hour). The weight of the container was noted for at least the next 2 hours, with the flow rate determined after a certain period of time (readiness level). Subsequently, without interruption of the initial flow, the infusion rate was doubled from 0.1 to 0.2, from 0.5 to 1.0 and from 1.0 to 2.0 ml/hour, respectively.
The flow of fluid was noted over the next hour. Each experiment was repeated 4 times on 2 identical Alaris Asena GH perfusors (8 measurements in total). A new sterile syringe was used for each experiment.
Data processing and statistical analysis
To balance the study, data were automatically recorded every 10 seconds and saved to a personal computer. To determine the mean effective time of readiness using the gravimetric method, a period of 60 min was used for each infusion rate (0.1, 0.5 and 1 ml/h) with an interval of 180 s [19]. The percentage coefficient of variation (CV) (standard deviation (SD)/mean x 100) was used to mathematically describe the change in flow [20]. Ready time and double volume infusion rate (measured from start to 95% ready) were measured at 180 sec intervals. Results were displayed as mean + SD. Two-way analysis of variables (ANOVA) followed by Bonferroni testing was used to determine the significance of differences between means. Statistical analysis was performed using Prism 2.0 (GraphPad software, San Diego, VA, USA); a P value of 0.05 was considered significant.
results
The average level of readiness, deviation from the average (%) and CI are presented in Table 1 .
Table 1. Availability time indicators
| Flow rate (ml/h) | Codan syringe | |||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 | 0.0986±0.0019 7.6 ± 4.8 98.6% | 0.0995±0.0016 13.4 ± 18.0 99.5% | 0.0997 ± 0.0029 44.7 ± 20.4*/y 99.7% | 0.1027±0.0040 4.8 ± 1.2 102.7%* |
| 0.5 | 0.4966 ± 0.0071 2.3 ± 0.9 99.3% | 0.5009±0.0041 3.8 ± 1.6 100.2% | 0.4990 ±0.0045 6.1 ± 2.2*/y 99.8% | 0.4963 ±0.0040 3.3 ± 1.4 99.3% |
| 1.0 | 0.9957 ± 0.0146 2.2 ± 0.9 99.6% | 0.9999 ±0.0083 2.9 ± 1.6 100.0% | 0.9995 ± 0.0053 3.3±6 1.2 100.0% | 1.0029 ± 0.0347 2.3 ± 1.1 100.3% |
| Syringe BD | ||||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 | 0.0996 ± 0.0013 2.9 ± 1.1 99.6% | 0.0983 ± 0.0020 2.8 ± 0.9 98.3% | 0.0990 ± 0.0022 3.3 ± 1.4 99.0% | 0.0916 ± 0.0042 3.3 ± 1.0 91.6%*/y |
| 0.5 | 0.5015 ± 0.0021 1.9 ± 1.0 100.3% | 0.4995 ± 0.0031 2.5 ± 1.3 99.9% | 0.4956 ± 0.0063 2.4 ± 1.4 99.1% | 0.4829 ± 0.0075 3.2 ±1.3 96.6% */y |
| 1.0 | 1.0045 ± 0.0038 2.0 ± 1.0 100.5% | 1.0081 ± 0.0058 2.6 ± 1.7 100.8% | 0.9981 ± 0.0057 2.4 ± 1.4 99.8% | 0.9817 ± 0.0116 2.6 ± 1.4 98.2% * |
Readiness parameters are presented in infusion rate (ml/h), CI and deviation of infusion rate from the declared one (%). Data are presented as mean ± SD
*Reflects the significance of statistical differences between different sizes of syringe of the same brand.
y Shows the significance of statistical differences between brands of syringe of a certain size (ANOVA). CI: coefficient of variability.
The measured liquid flow in the ready state was within 2% of standard except for the BD 50-ml, where the deviation was up to 9% at a flow of 0.1 ml/hour and 4% at a flow of 0.5 ml/hour. The deviation of the actual flow rate from the declared one was influenced by the syringe size (p <0.05), syringe brand (at a flow of 0.1 and 0.5 ml/hour, p <0.01) and flow rate (for BD syringes, p < 0.0001). CI was significantly higher for Codan syringes compared to BD (p < 0.001) and was also dependent on flow rate (p < 0.05) and syringe size (for Codan syringes, p < 0.0001). The Codan 30 ml syringe in particular showed the highest CI: up to 50%.
The readiness time of the syringe system after administering a 1 ml bolus varied from 3.6 + 0.9 min (BD 10 ml at a speed of 1 ml/hour) to 74.5 + 26.6 min (BD 50 ml at a speed of 0.1 ml\ hour) ( Table 2, Figure 1 ). Overall, start time was highly dependent on flow rate (0.1 ml/h, 1 ml/h, P < 0.0001), syringe size (50 ml and 10 ml, P < 0.01), and syringe brand (BD and Codan, P < 0.01). . For BD syringes, the effect of size was greater at low flows than at high flows (two-way ANOVA: factor 1 (flow rate), factor 2 (syringe size) and factor interaction (P<0.0001).
Table 2. Ready time.
| Infusion rate (ml/h) | Codan syringe – ready time (min) | |||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 | 12.3 ± 3.8 | 22.6 ± 7.9* | 15.4 ± 8.3 | 20.4 ± 10.4 |
| 0.5 | 4.8 ± 2.0 | 6.9 ± 4.8 | 6.1 ± 2.8 | 7.7 ± 3.4 |
| 1.0 | 3.9 ± 1.7 | 4.1 ± 0.8 | 3.9 ± 0.5 | 5.3 ± 2.1 |
| Syringe BD ready time (min) | ||||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 | 20.5 ± 10.8 | 25.2 ± 15.6 | 27.3 ± 10.1 | 74.5 ± 26.6*/y |
| 0.5 | 4.1 ± 1.0 | 9.0 ± 3.0 | 8.5 ± 3.1 | 18.3 ± 11.8*/y |
| 1.0 | 3.6 ± 0.9 | 6.2 ± 2.9 | 4.8 ± 1.5 | 13.9 ± 7.4*/y |
Ready Time – Ready for a stable infusion. Data are presented as mean ± SD.
*Reflects the significance of statistical differences compared to smaller syringes of the same brand.
y Reflects the significance of statistical differences between different brands of syringes of a certain size (ANOVA).
| Fig.1. Ready times for various Codan and BD syringes (10, 20, 30 and 50 ml) at infusion rates of 0.1, 0.5 and 1.0 ml/h. Data are presented as mean ± SD. * P < 0.05 compared with small syringes of the same brand at the same infusion rate y P < 0.001 compared with Codan syringes of the same size at the same flow rate. # P <0.01 comparing syringes of the same size and brand at a higher infusion rate. |
The time to stable flow rate at twice the volume varied from 3.0 to 5.0 + 1.4 minutes for the Codan syringe and from 3.0 to 12.8 + 3.7 minutes for the BD syringe, and was mainly dependent on the flow rate (P < 0.01) and the brand of syringe (P < 0.01). ( Table 3, Fig. 2 ). For BD syringes, flow rate at doubling was highly dependent on syringe size, which occurred at low infusion rates (two-way ANOVA: factor 1 (flow rate), factor 2 (syringe size), and interaction between factors (P < 0.0001).
Table 3. Double infusion rate
| Speed inf. (ml/h) | Codan syringe – ready time (min) | |||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 — 0.2 | 3.0 ± 0.1 | 4.2 ± 2.4 | 3.0 ± 0.1 | 5.0 ± 1.4 |
| 0.5 — 1.0 | 3.0 ± 0.0 | 3.1 ± 0.1 | 3.8 ± 0.9 | 3.2 ± 0.3 |
| 1.0 — 2.0 | 3.0 ± 0.0 | 3.1 ± 0.1 | 3.0 ± 0.4 | 3.0 ± 0.1 |
| BD Syringe - Ready Time (min) | ||||
| 10 ml | 20 ml | 30 ml | 50 ml | |
| 0.1 — 0.2 | 3.2 ± 0.4 | 4.5 ± 1.3 | 6.8 ± 0.6 | 12.8 ± 3.7*/y |
| 0.5 — 1.0 | 3.3 ± 0.6 | 3.0 ± 0.1 | 3.6 ± 0.4 | 5.6 ± 1.6*/y |
| 1.0 — 2.0 | 3.0 ± 0.0 | 3.1 ± 0.1 | 3.3 ± 0.4 | 3.3 ± 0.1 |
Ready time at double infusion rate. Data are presented as mean ± SD
*Reflects the significance of statistical differences compared to smaller syringes of the same brand.
y Reflects the significance of statistical differences between different brands of syringes of a certain size (ANOVA).
| Fig.2. Double the infusion rate for various Codan and BD syringes (10, 20,30 and 50 ml) by changing the rate from 0.1 to 0.2, 0.5 to 1.0 and from 1.0 to 2.0 ml/h. Data are presented as mean ± SD. *P < 0.01 compared with small BD syringes at the same infusion rate. y P < 0.001 compared to Codan syringes of the same size at the same infusion rate. # P < 0.05 comparing syringes of the same size and brand at a higher infusion rate. |
Discussion
Critically ill patients often require simultaneous and continuous infusions of various drugs, such as vasoactive drugs, sedatives, and analgesics.
In particular, in neonates and children, it is necessary to infuse at a low rate of 0.5-1.0 ml/h to avoid fluid overload.
Our results show that continuous fluid infusion is very accurate through both brands of syringes, with the exception of the BD 50 ml. Unexpectedly, fluctuating flow (FI) was higher with Codan compared to BD. The reason for this may be the plunger in the Codan syringe, which is made of silicone, and this is the only inconvenience of this type of syringe. The syringe plunger phenomenon has been previously described by Capes et al for other brands of syringes [7]. Ready time and doubling flow rate clearly and significantly decrease as the syringe size decreases. This effect was particularly pronounced at a very low flow rate (0.1 ml/h), and disappeared at a higher flow rate (1.0 ml/h).
In a situation requiring immediate hemodynamic support (emergency) or during a procedure, a constant flow of fluid is desirable when changing the syringe. Subsequently, the start time and increase time of fluid flow (in the present study defined as twice the flow time) should be kept as short as possible, particularly in hemodynamically unstable patients.
Our data show that ready time varies significantly with syringe size, especially at low infusion rates. A significant delay in readiness of up to 75 minutes for a BD 50 ml syringe at a low infusion rate is a common occurrence, even despite filling the syringe before connecting. Moreover, it has been found that other factors such as air bubbles in the syringe, flow direction valves and other resistance elements can increase the delay [21,22]. The significant onset delay shown in our study unpredictably delays the desired drug effect, whereas reducing syringe size, optimizing syringe shape, using stiffer materials, and high infusion rates significantly improve onset performance. It is a fact that a smaller piston diameter or a less compressible piston made of a rigid, incompressible material will reduce the compressibility of the system as a whole, and a high infusion rate will overcome the remaining system resistance. Interestingly, Codan syringes generally exhibited less variation in ready times among syringe sizes tested compared to BD syringes. The explanation for this may be the flat and relatively incompressible piston of the Codan syringe, which is adjacent to the walls only with a silicone ring. Conversely, the BD piston is made of silicone, which makes it more compressible. This is consistent with previous findings of decreased sensitivity to the vertical position of Codan syringe perfusors [12]. In the past, so-called FASTSTARTs capability (NAC P7000 Perfusor; Alaris Medical Systems, Hampshire, UK) was available for some perfusers, which significantly reduced turnaround time [23]. However, FASTSTARTs require a special infusion line that does not fit conventional perfusers, and a priming technique has been proposed to improve efficiency. Changing the syringe with vasoactive drugs is often critical for patients, as instability of the cardiovascular system can occur. The so-called double infusion or combination is a common technique to reduce unwanted changes in plasma drug concentrations when changing the syringe. In this method, a parallel infusion of the same drug is given, and when one syringe runs out, an infusion from another syringe replaces the drug through a 3-way stopcock. However, replacement may be problematic in patients with unstable blood pressure (hypotension, hypertension) and tachycardia. In this case, improved readiness characteristics will significantly improve hemodynamic stability and patient safety [24–26]. The 50ml infusion syringe is most commonly used in adults and children.
If necessary, change each syringe once a day according to microbiological rules, while maintaining an infusion rate of 0.5 ml/h, it is sufficient to use a 20 ml syringe. This will significantly improve syringe performance at the start of infusion and significantly reduce the effect of hydrostatic pressure when placing the perfuser in a vertical position, while reducing the response time of the perfuser to occlusion [27-29].
Moreover, the use of small syringes reduces the consumption of drugs, reducing the cost of treatment. If the situation requires the use of a 50 ml syringe, it is preferable to use Codan syringes, which reduce the readiness time at the start and after increasing the infusion rate.
Some limitations of the study need to be taken into account. Fluid infusion was studied using only one brand of perfusor. In a clinical setting, other factors may also influence the start-up time and when changing the infusion rate. These include, for example, the length of the infusion line if it is not pre-filled with a drug solution, ignoring the initial filling of the line, changes in hydrostatic pressure (for example, if the perfuser is located below the tip of the catheter, with a high central venous pressure or under the influence of a hydrostatic column of liquid during simultaneous infusion into the same catheter port), increasing resistance when using filters, using very thin silicone catheters in newborns, using a viscous fat solution and parallel infusion into the same catheter port, which increases the overall resistance of the system. All these factors were not considered in our study.
Despite the large differences in the characteristics of different perfusers, further improvements in technology, such as optimization of syringe distensibility, improvement of perfusors and motors, will overcome many of the limitations of modern perfusers. However, a new generation of dosing devices is needed. Recently, new perfusers (micro-dosing devices) have become available that may revolutionize infusion technology and set new standards for patient safety [30]. Manufacturers of infusion devices are trying to solve the problems associated with them and make the new generation a modern and safe infusion device.
In conclusion, syringe size and syringe type significantly influence readiness characteristics at low infusion rates. To improve the performance of perfusers, it is preferable to use small syringes. Moreover, there is a need to improve syringe design, physical characteristics, and compatibility with perfusers. In order to improve the performance of perfusers and therefore patient safety, it is necessary to introduce modern infusion perfusors in the future.
Bibliography:
- Rooke GA, Bowdle TA. Syringe pumps for infusion of vasoactive drugs: mechanical idiosyncrasies and recommended operating procedures. Anesth Analg 1994; 78: 150-156.
- Shibata H, Aibiki M, Shirakawa Y, Ogli K. Dopamine infused continuously at high concentration with a low flow rate affects arterial blood pressure fluctuation waves. Crit Care Med 1993; 21: 801-804.
- Klem SA, Farrington JM, Leff RD. Influence of infusion pump operation and flow rate on hemodynamic stability during epinephrine infusion. Crit Care Med 1993; 21: 1213-1217.
- Krauskopf KH, Rauscher J, Brandt L. Disturbance of continuous, pump administration of cardiovascular drugs by hydrostatic pressure. Anaesthesist 1996; 45: 449-452.
- Schulze KF, Graff M, Schimmel MS, Schenkman A, Rohan P. Physiologic oscillations produced by an infusion pump. J Pediatr 1983; 103: 796-798.
- Leff RD, Roberts RJ. Problems in drug therapy for pediatric patients. Am J Hosp Pharm 1987; 44: 865-870.
- Capes DF, Dunster KR, Sunderland VB, McMillan D, Colditz PB, McDonald C. Fluctuations in syringe-pump infusions: association with blood pressure variations in infants. Am J Health Syst Pharm 1995; 52: 1646-1653.
- Lonnqvist PA, Lofqvist B. Design flaw can convert commercially available continuous syringe pumps to intermitent bolus injectors. Intens Care Med 1997; 23: 998-1001.
- Cunningham S, Deere S, McIntosh N. Cyclical variation of blood pressure and heart rate in neonates. Arch Dis Child 1993; 69: 64-67.
- Lonnqvist PA. How continuous are continuous drug infusions? Intens Care Med 2000; 26: 660-661. eleven.
- Weiss M, Hug MI, Neff T, Fischer J. Syringe size and flow rate affect drug delivery from syringe pumps. Can J Anaesth 2000; 47: 1031-1035.
- Weiss M, Fischer J, Neff T, Baenziger O. The effects of syringe plunger design on drug delivery during vertical displacement of syringe pumps. Anaesthesia 2000; 55: 1094-1098.
- Weiss M, Banziger O, Neff T, Fanconi S. Influence of infusion line compliance on drug delivery rate during acute line loop formation. Intens Care Med 2000; 26: 776-779.
- Neff TA, Fischer JE, Schulz G, Baenziger O, Weiss M. Infusion pump performance with vertical displacement: effect of syringe pump and assembly type. Intens Care Med 2001; 27: 287-291.
- Kern H, Kuring A, Redlich U et al. Downward movement of syringe pumps reduces syringe output. Br J Anaesth 2001; 86: 828-831.
- Igarashi H, Obata Y, Nakajima Y, Katoh T, Morita K, Sato S. Syringe pump displacement alters line internal pressure and flow. Can J Anaesth 2005; 52: 685-691.
- Cook RI. Syringe pump assemblies and the natural history of clinical technology. Can J Anaesth 2000; 47: 929-935.
- Neff T, Fischer J, Fehr S, Baenziger O, Weiss M. Start-up delays of infusion syringe pumps. Paediatr Anaesth 2001; 11: 561-565.
- Leff RD, True WR, Roberts RJ. A gravimetric technique for evaluating flow continuity from two infusion devices. Am J Hosp Pharm 1987; 44: 1388-1391.
- Stull JC, Erenberg A, Leff RD. Flow rate variability from electronic infusion devices. Crit Care Med 1988; 16: 88-891.
- Schulz G, Fischer J, Neff T, Banziger O, Weiss M. The effect of air within the infusion syringe on drug delivery of syringe pump infusion systems. Anaesthesist 2000; 49: 1018-1023.
- McCarroll C, McAtamney D, Taylor R. Alteration in flow delivery with antisyphon devices. Anaesthesia 2000; 55: 355-357.
- Neff T, Fischer J, Fehr S, Baenziger O, Weiss M. Evaluation of the FASTSTART mode for reducing start-up delay in syringe pump infusion systems. Swiss Med Wkly 2001; 131: 219-222.
- 24. Amoore J, Dewar D, Ingram P, Lowe D. Syringe pumps and start-up time: ensuring safe practice. Nurs Stand 2001; 15: 43-45.
- Powell ML, Carnevale FA. A comparison between single and double-pump syringe changes of intravenous inotropic medications in children. Dynamics 2004; 15:10-14.
- Trim JC, Roe J. Practical considerations in the administration of intravenous vasoactive drugs in the critical care setting: the double pumping or piggyback technique-part one. Intens Crit Care Nurs 2004; 20: 153-160.
- Kim DW, Steward DJ. The effect of syringe size on the performance of an infusion pump. Paediatr Anaesth 1999; 9: 335-337.
- Weiss M, Neff T, Gerber A, Fischer J. Impact of infusion line compliance on syringe pump performance. Paediatr Anaesth 2000; 10: 595-599.
- Donmez A, Araz C, Kayhan Z. Syringe pumps take too long to give occlusion alarm. Paediatr Anaesth 2005; 15: 293-296.
- Weiss M, Gerber S, Fuchslin RM, Neff TA. Accurate continuous drug delivery at low infusion rate with a novel microvolumetric infusion pump (MVIP): pump design, evaluation and comparison to the current standard. Anaesthesia 2004; 59: 1133-1137.