Currently, acute cerebrovascular accidents are the most common life-threatening disease and one of the main causes of disability not only in Russia, but also in the world. According to the World Federation of Neurological Societies, about 15 million strokes are registered annually in the world. In Russia, the incidence of stroke is 3.4 per 1000 people per year, with about 30% of strokes leading to death in the acute period [1, 2]. Over the next year, another 10-15% of patients die. 80% of stroke survivors develop some degree of limitation in activities of daily living [3]. In our country, disability due to stroke (3.2 per 10,000 population per year) is ahead of other diseases [4].
A significant negative contribution to the disability of patients after a stroke is made by cognitive impairment, since often, in the absence of clear focal symptoms, it is the cognitive impairment that determines the severity of the patient’s condition and difficulties in the process of his everyday and professional adaptation. During the first year after an ischemic stroke, dementia develops in 25–30% of cases [5]. In approximately 1/3 of cases, it is the result of decompensation or activation of a previous neurodegenerative process; in other cases, post-stroke dementia is caused by cerebral vascular insufficiency [6-8]. In patients with post-stroke dementia, the risk of developing a recurrent stroke increases, which may be due to the difficulties of secondary prevention in this group of patients. There is evidence that the presence of severe cognitive deficits also negatively affects the restoration of motor functions [9–12].
A special form of cognitive disorders is aphasia, which is a disorder of formed speech that occurs with focal lesions of the cortex and adjacent subcortical region of the dominant hemisphere of the brain and is a systemic disorder of various forms of speech activity while maintaining the elementary forms of hearing and movements of the speech apparatus [13]. Total and sensory aphasia in the acute period is observed in 84% of patients with hemorrhages and in 50% with ischemic stroke in case of damage to the dominant hemisphere; after several months, sensorimotor aphasia persists in 12-13% of patients [6]. Any form of cognitive impairment in general and speech in particular leads to personality changes and disruption of general and verbal communication. The patient's behavior, emotional state, and quality of life change.
The use of neuroprotective drugs in the acute period of stroke has been widely discussed for a long time. Most researchers are inclined to believe that early administration of neuroprotectors can improve the prognosis for the recovery of impaired functions, increase the period of the “therapeutic window,” and reduce the size of the focus of brain damage [14–16]. Currently, there is an active search for effective drugs for primary and secondary neuroprotection, and studies are being conducted to prove the need to prescribe these drugs in the acute period of stroke.
Currently, a new original neuroprotective drug for parenteral administration has been developed - Cellex (Pharm-Sintez CJSC, Russia). It is a protein-peptide complex from the embryonic brain of pigs, which is capable of ensuring the regeneration of human nervous tissue. Cellex has a direct neuroreparative effect, which fundamentally distinguishes it from neurotropic drugs from other groups. The direct reparative effect of Cellex on neuronal and glial cell pools of nervous tissue has been proven by preclinical studies: in experiments with laboratory animals on models of acute ischemic brain injury using photoinduced thrombosis, on cell cultures of cerebellar neurons in a model of glutamate toxicity, as well as in models of global transient and incomplete hypoxia brain
The results obtained were the basis for conducting a clinical study “Multicenter randomized comparative open clinical trial of the effectiveness and safety of the drug Cellex in the treatment of patients with acute cerebrovascular accident,” which included studying the effect of Cellex on cognitive and speech disorders.
The purpose of the study was to study the effect of Cellex on the restoration of cognitive and speech functions in the acute period of stroke.
Material and methods
A multicenter comparative open clinical study of the effectiveness and safety of the drug Cellex in the treatment of patients with acute stroke was conducted at 6 clinical sites. The study included 180 stroke patients. The diagnosis of acute cerebrovascular accident, the nature and location of the stroke were established according to the criteria of the stroke registry of the National Stroke Association (NABI) [5], based on medical history (previous diseases of the cardiovascular system, blood pressure, the nature of the development of neurological symptoms), clinical data (neurological examination assessing the severity of cerebral, meningeal and focal symptoms) and additional research data (laboratory tests of blood and cerebrospinal fluid, fundus examination, computed tomography or magnetic resonance imaging of the brain, transcranial Doppler sonography and duplex scanning of cerebral vessels with embolodetection ).
Criteria for inclusion of patients in this study: men aged 35 to 80 years inclusive; women aged 55 to 80 years inclusive, who have been postmenopausal for at least 2 years; patients who have signed written informed consent or their closest relatives (if the patient is extremely ill); patients with acute ischemic or hemorrhagic stroke in accordance with ICD-10 criteria, confirmed by X-ray computed tomography (CT) or magnetic resonance imaging (MRI) of the brain, hospitalized within 1 day from the onset of the disease.
The exclusion criteria were: extreme severity of the patient with a level of consciousness below 5 points on the Glasgow Coma Scale (coma stage III according to the Russian classification); regression of neurological symptoms within the first 24 hours from the onset of the disease (transient ischemic attack); the patient has diseases or conditions indicated as contraindications to the use of the drug Cellex: epilepsy, manic psychosis, productive delirium, delirium; a history of anaphylactic reactions to drugs of a protein nature (albumin, blood plasma, immunoglobulins, serum); the presence of a malignant neoplasm; acute and/or chronic bacterial and viral diseases in the acute stage; autoimmune diseases in the stage of decompensation, accompanied by systemic vasculitis; chronic liver or kidney failure in the acute stage; severe or uncontrolled heart pathology; previous neurotropic metabolic therapy within 14 days before the patient's hospitalization.
Among the patients included in the study, 147 (81.7%) suffered ischemic stroke and 33 (18.3%) suffered hemorrhagic stroke.
It can also be added to the characteristics of the sample that 148 (82%) patients showed signs of atherosclerosis. In 135 (75%) cases, a combination of atherosclerosis and arterial hypertension was noted. In 158 (87.8%) cases there was hypertension. Diabetes mellitus, combined with atherosclerosis and arterial hypertension, was detected in 14 (7.8%) patients. In 78 (43.3%) cases, signs of coronary heart disease were determined, 15 (8.3%) patients had previously suffered a myocardial infarction. 37 (20.5%) patients had a permanent form of atrial fibrillation: against the background of coronary artery sclerosis - in 29, against the background of rheumatism - in 8; 8 (4.4%) patients had previously suffered a stroke in the same vascular territory, of which in 3 this was preceded by transient ischemic attacks. All patients were admitted to the hospital within the first day of illness.
Cellex was used against the background of standard basic therapy in the form of a solution for subcutaneous administration of 0.1 mg 1 time per day for the first 10 days and from the 21st to the 27th day of the disease. All drugs that are not included in standard basic therapy and have neurotropic, antihypoxic effects were prohibited for use throughout the entire study period. In cases where the use of these drugs against the background of basic therapy was unavoidable, the patient was excluded from the study.
Depending on the characteristics of therapy, patients were divided into two groups - the main group and the comparison group.
The main group of 180 included 90 (50%) patients who were prescribed Cellex as part of basic therapy. Their average age was 61.4±9.5 years. The comparison group also consisted of 90 (50%) patients who received only basic therapy. Their mean age was 62.2±8.9 years.
In addition, depending on the severity of the condition, patients in the main group and the comparison group were further divided into three subgroups. When determining the severity of the condition, the severity of changes in consciousness and other cerebral symptoms, meningeal symptoms, vegetotrophic disorders, and focal neurological disorders at the time of admission to the clinic were taken into account. The severity of the condition was divided according to the Stroke Scale (NIHSS). Mild degree corresponded to 0-6 points, moderate - 7-14 points, severe - 15 points and above.
In the main group, mild severity of the condition was determined in 21 (23.3%) patients, moderate in 49 (54.4%) patients and severe in 20 (22.2%). In the comparison group, the degree was mild in 36 (40%) cases, moderate in 44 (49%) and severe in 10 (11%).
The distribution of patients in the selected groups and subgroups by age, gender, severity, nature and area of injury is presented in Table. 1 and 2.
Table 1. Distribution of patients in the main group and the comparison group by gender, type of stroke, severity of condition and area of damage in the Cellex group Note. LMCA—left middle cerebral artery; PSMA—right middle cerebral artery; VBB - vertebrobasilar system; LA - left hemisphere; PP - right hemisphere; SAH - subarachnoid hemorrhage.
Table 2. Distribution of patients in the studied groups according to indicators of the state of higher mental functions
All patients were monitored for basic physiological indicators (blood pressure, heart rate, electrocardiography, respiratory rate, body temperature, glycemia) in the first 48 hours from the onset of stroke 4-6 times a day, depending on the severity of the patient’s condition. Correction and maintenance of hemodynamics, respiration, water-electrolyte metabolism and glucose metabolism, correction of cerebral edema and increased intracranial pressure, adequate nutritional support, prevention and control of complications were also carried out.
The dynamics of impairment of higher cortical functions was assessed using the TIPC test (Information—Memory—Concentration of Attention) and OR (speech questionnaire). The level of preservation of consciousness of patients in the acute period of stroke was also assessed in accordance with the Glasgow Coma Scale: values of 8 points and below indicated the presence of coma, 9-12 points - from stupor to stupor, 13-15 points - from mild retardation to clear consciousness.
Statistical processing of the obtained results was carried out using the Microsoft Excel 2003 and Statistica 6.0 software packages using the ANOVA and DENOVA packages. If the characteristic was normally distributed, t
-Student's test and analysis of variance with repeated measurements, when the distribution of a sign is different from normal - rank analysis of variance, Wilcoxon sign tests and pairwise comparisons, for testing dependence - Fisher's exact test.
Differences were considered statistically significant at p
<0.05.
168 patients completed the full 4-week study; 4 patients were withdrawn for objective reasons: detection of metastatic brain damage, development of delirium tremens, transfer to the neurosurgical department for surgical treatment of intracerebral hematoma, transfer to the hospital of another hospital at the insistence of relatives. Of the 3 patients in the comparison group who died, 1 patient died as a result of pulmonary embolism, 1 patient developed acute myocardial infarction against the background of paroxysmal atrial fibrillation, 1 patient had an increase in cerebral and focal neurological symptoms with cerebral edema by the 3rd day. Of the 5 patients who died who received Cellex, 1 patient had a pathological diagnosis of a brain tumor, 1 patient admitted in extremely serious condition had an increase in edema with herniation of the brain stem by the 3rd day, 3 patients admitted in serious condition , there was a progressive increase in focal neurological deficit and cerebral symptoms with decompensation of concomitant pathology (in 1 patient - type 2 diabetes mellitus and atrial fibrillation, in 1 patient - liver failure against the background of chronic alcoholism, in 1 patient - pronounced progression of cardiovascular failure due to pacemaker failure).
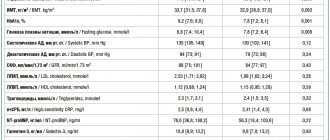
When analyzing the state of higher mental functions on clinical scales (average NIHSS score; average score for disorders of consciousness, average OR values), the compared groups initially differed significantly: the state of these functions in patients treated with Cellex was initially significantly more severe than in the comparison group (see Table 2).
The assessment of the dynamics of neurological deficit on clinical scales from day 1 to days 3, 6, 10, 21 and 28 was carried out by comparing the average changes in clinical scores in the study groups using the Student t-test for independent samples. The study of the significance of differences between indicators at control points was carried out using the method of analysis of variance.
Cellex®
Manufacturer: PHARM-SINTEZ CJSC (Russia)
solution d/s/c administration 0.1 mg/1 ml: amp. 1 ml or 2 ml 1, 2, 4, 5 or 10 pcs. Reg. No.: LP-001393
Clinical and pharmacological group:
Nootropic drug
Release form, composition and packaging
Solution for subcutaneous administration
transparent, colorless or light yellow, odorless or with a specific odor; Opalescence is allowed.
| 1 ml | |
| Cellex® substance-solution (frozen, expressed as protein*) | 0.1 mg |
Excipients:
glycine - 3.75 mg, sodium hydrogen phosphate dihydrate - 2.99 mg, sodium dihydrogen phosphate monohydrate - 0.47 mg, sodium chloride - 5.85 mg, liquid water - up to 1 ml.
* Composition per 1 ml. Active substance:
polypeptides from the brain of pig embryos, the active substance in terms of total protein is 0.9-2.4 mg (nominal content of total protein is 1.65 mg per 1 ml of substance).
Excipients:
glycine - 3.75 mg, 0.1M disodium hydrogen phosphate solution - up to pH 7.8 (about 0.8 mg of dry matter), sodium chloride - 5.85 mg, polysorbate 80 - 0.005 mg, purified water - up to 1 ml.
1 ml - dark glass ampoules (1) - contour cell packaging made of polyvinyl chloride film (1) - cardboard packs. 1 ml - dark glass ampoules (1) - contour cell packaging made of polyvinyl chloride film (2) - cardboard packs. 1 ml - dark glass ampoules (2) - contour cell packaging made from polyvinyl chloride film (1) - cardboard packs 1 ml - dark glass ampoules (2) - contour cell packaging made from polyvinyl chloride film (2) - cardboard packs. 1 ml - dark glass ampoules (5) - contour cell packaging made of polyvinyl chloride film (1) - cardboard packs. 1 ml - dark glass ampoules (5) - contour cell packaging made of polyvinyl chloride film (2) - cardboard packs. 2 ml - dark glass ampoules (1) - contour cell packaging made of polyvinyl chloride film (1) - cardboard packs. 2 ml - dark glass ampoules (1) - contour cell packaging made of polyvinyl chloride film (2) - cardboard packs. 2 ml - dark glass ampoules (2) - contour cell packaging made of polyvinyl chloride film (1) - cardboard packs. 2 ml - dark glass ampoules (2) - contour cell packaging made of polyvinyl chloride film (2) - cardboard packs. 2 ml - dark glass ampoules (5) - contour cell packaging made of polyvinyl chloride film (1) - cardboard packs. 2 ml - dark glass ampoules (5) - contour cell packaging made of polyvinyl chloride film (2) - cardboard packs.
Description of the active components of the drug "Cellex®"
pharmachologic effect
Nootropic drug. The presence of tissue-specific signaling proteins and polypeptides - growth factors, nerve cell differentiation factors in the drug determines its direct neuroreparative effect, due to the regulation of the concentrations of the pool of neurotransmitters, with inhibition of the spillover of excitatory amino acids.
The drug activates secondary neuroprotection by stimulating synaptogenesis processes, restoring autophagy signals, improving tissue immunoregulation with inhibition of immunogenic cytotoxicity of macrophages. At the same time, a tissue-specific and systemic reparative effect of the drug is noted with the restoration of the regenerative and reparative potential of brain cells, a decrease in the number of damaged cells and the severity of perifocal edema in the penumbra zone (allows for significant limitation of the focus of brain tissue necrosis) with the restoration of microcirculation and general perfusion.
Restoration and regulatory stimulation of various compartments of the central nervous system with the systemic influence of growth factors, differentiation and signaling molecules ensures a reduction in the time of recovery and rehabilitation of patients with damage to the central and peripheral nervous system of vascular origin and restoration of motor, sensory and cognitive functions.
The therapeutic effect usually develops 3-5 days after the start of drug administration.
Indications
Cerebrovascular diseases:
—
acute cerebrovascular accidents in the acute and early rehabilitation period of the disease as part of complex therapy.
Dosage regimen
For adults
the drug is prescribed in a dose of 0.1-0.2 mg 1 time/day subcutaneously for 10 days, depending on the severity of the patient’s condition. If necessary, repeat the course after 10 days.
Studies on the use of the drug in children
were not carried out.
Side effect
Maybe:
allergic reactions in the form of mild hyperemia at the injection site, hypersensitivity reactions (skin rash, itching, angioedema), low-grade fever, sleep disturbance, headache.
Contraindications
- epilepsy;
- manic psychosis;
- productive delirium;
- delirium;
- age under 18 years (due to insufficient clinical data).
Carefully _
The drug should be prescribed if there is a history of allergic reactions to drugs of a protein-peptide nature.
Pregnancy and lactation
During pregnancy, the effect of the drug has not been studied. The lack of appropriate studies does not allow the drug to be used in this group of patients.
Application for children
Studies on the use of the drug in pediatric practice have not been conducted.
special instructions
The drug should be prescribed with extreme caution for malignant arterial hypertension in the stage of decompensation, sympatho-adrenal crises such as panic attacks, severe anxiety-depressive disorders.
The drug does not contain prion infections or viruses.
Impact on the ability to drive vehicles and operate machinery
Currently, there is no data on the effect of the drug Cellex® on the ability to drive a car and operate machinery, which requires increased attention and speed of mental and motor reactions.
Overdose
Currently, there are no cases of overdose of Cellex®.
Drug interactions
When used together with psychostimulant drugs and alcohol, psychomotor agitation and sleep disturbances are possible.
It is possible to reduce the activity of anesthetics, tranquilizers, and antipsychotics.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
The drug should be stored in a place protected from light, out of reach of children, at a temperature of 2° to 8°C. Shelf life: 1 year.
Drug interactions
When used together with psychostimulant drugs and alcohol, psychomotor agitation and sleep disturbances are possible.
It is possible to reduce the activity of anesthetics, tranquilizers, and antipsychotics.
Cellex solution SC injection 0.1 mg/ml 1 ml amp N 5
Active substance:
Polypeptides from fetal pig brain
ATX
N06BX Other psychostimulants and nootropic drugs
Pharmacological groups
- Nootropic drugs, other drugs affecting the nervous system [Nootropics]
- Nootropic drugs, other drugs affecting the nervous system [Other neurotropic drugs]
Compound
| Solution for subcutaneous administration | 1 ml |
| active substance: | |
| Cellex® substance-solution (frozen in terms of protein1) | 0.1 mg |
| excipients: glycine - 3.75 mg; sodium hydrogen phosphate dihydrate - 2.99 mg; sodium dihydrogen phosphate monohydrate - 0.47 mg; sodium chloride - 5.85 mg; water for injection - up to 1 ml | |
| 1 composition per 1 ml: active substance - polypeptides from the brain of pig embryos, in terms of total protein - 0.9–2.4 mg (nominal content of total protein - 1.65 mg per 1 ml of substance); excipients - glycine - 3.75 mg, 0.1 M disodium hydrogen phosphate solution - up to pH 7.8 (about 0.8 mg of dry matter), sodium chloride - 5.85 mg, polysorbate 80 - 0.005 mg, purified water - up to 1 ml |
Description of the dosage form
Solution: transparent, colorless or light yellow liquid, odorless or with a specific odor. Opalescence is allowed. The presence of separate threads of coagulum is allowed.
pharmachologic effect
Pharmacological action - neuroprotective.
Pharmacodynamics
The presence of tissue-specific signaling proteins and polypeptides - growth factors, differentiation of nerve cells in the drug - determines its direct neuroreparative effect by regulating the concentrations of the pool of neurotransmitters with inhibition of spillover of excitatory amino acids.
The drug activates secondary neuroprotection by stimulating synaptogenesis processes, restoring autophagy signals, improving tissue immunoregulation with inhibition of immunogenic cytotoxicity of macrophages. At the same time, a tissue-specific and systemic reparative effect of the drug is noted with the restoration of the regenerative and reparative potential of brain cells, a decrease in the number of damaged cells and the severity of perifocal edema in the penumbra zone (allows for significant limitation of the focus of brain tissue necrosis) with the restoration of microcirculation and general perfusion.
Restoration and regulatory stimulation of various compartments of the central nervous system with the systemic influence of growth factors, differentiation and signaling molecules ensures a reduction in the time of recovery and rehabilitation of patients with damage to the central nervous system and peripheral nervous system of vascular origin and restoration of motor, sensory and cognitive functions. The therapeutic effect usually develops 3–5 days after the start of drug administration.
Pharmacokinetics
The complex composition of the drug Cellex®, the active fraction of which consists of a balanced and stable mixture of biologically active proteins and polypeptides with a total polyfunctional effect, does not allow for conventional pharmacokinetic analysis of individual components.
Indications for Cellex®
Cerebrovascular diseases are acute disorders of cerebral circulation in the acute and early rehabilitation period of the disease, as part of complex therapy.
Contraindications
epilepsy;
manic psychosis;
productive delirium;
delirium;
age under 18 years (due to insufficient clinical data).
With caution: a history of allergic reactions to drugs of a protein-peptide nature.
Use during pregnancy and breastfeeding
The lack of appropriate studies does not allow the drug to be used in this group of patients.
Side effects
Allergic reactions may occur in the form of mild hyperemia at the injection site, hypersensitivity reactions (skin rash, itching, angioedema), low-grade fever, sleep disturbance, headache.
Interaction
When used together with psychostimulant drugs and alcohol, psychomotor agitation and sleep disturbances are possible.
It is possible to reduce the activity of anesthetics, tranquilizers, and antipsychotics.
Directions for use and doses
PC.
Adults: 0.1–0.2 mg 1 time per day subcutaneously for 10 days, depending on the severity of the patient’s condition. If necessary, repeat the course after 10 days. Studies on the use of the drug in pediatric practice have not been conducted.
Rules for introducing the solution
The drug is administered subcutaneously through a sterile syringe filter included in the kit.
For administration, draw the required amount of Cellex® into the syringe, remove the needle, then put a sterile syringe filter with a pore diameter of 0.22 microns on the syringe. Take a new needle and place it on a sterile syringe filter. The drug is ready for use.
Overdose
Currently, there are no cases of overdose of Cellex®.
special instructions
It is prescribed with special caution for arterial hypertension of a malignant course in the stage of decompensation, sympathoadrenal crises such as panic attacks, severe anxiety-depressive disorders.
During pregnancy, the effect of the drug has not been studied.
The drug does not contain prion infections and viruses.
Impact on the ability to drive vehicles and operate machinery. Currently, there is no data on the effect of the drug Cellex® on the ability to drive a car and work with mechanisms that require increased attention and speed of mental and motor reactions.
Release form
Solution for subcutaneous administration, 0.1 mg/ml. In dark glass ampoules with a capacity of 1 or 2 ml, imported, with a tension ring for opening, or in ampoules with a breaking point, 1 or 2 ml. Color marking of ampoules in the form of two green stripes is allowed. 1, 2, 5 amp. 1 or 2 ml in blister packs made of PVC film. 1 or 2 blister packs in a cardboard pack. Each pack contains sterile syringe filters with a pore diameter of 0.22 microns in an amount equal to the number of ampoules.
Conditions for dispensing from pharmacies
On prescription.
Cellex
Cellex is a drug from the nootropic group. Includes tissue-specific proteins that transmit signals between cells, polypeptide growth factors, and neuronal differentiation factors. Has a neuroreparative effect. Regulates the content and ratio of neurotransmitters with suppression of the diffusion of excitatory amino acids. Stimulates the processes of origin and development of synapses. Restores signals that initiate autophagy. Improves immunoregulation at the cell and tissue level. Suppresses the toxicity of histophagocytes to cells. Promotes the regeneration and repair of brain cells, protects them from damage. Reduces perifocal edema in the ischemic penumbra zone. Limits the focus of brain tissue necrosis. Restores microcirculation in the cerebral cortex, promotes blood saturation of cerebral tissues. The therapeutic effect of taking the drug usually develops 3-5 days after the start of the drug course. Frequency of application – 1 time per day. The duration of treatment is on average 10 days, depending on the clinical situation and the severity of the therapeutic response. It is possible to conduct a repeat course, which is carried out after a 10-day break. The drug is not used in pediatric practice due to the lack of clinical data on its effectiveness and safety in this category of patients. Cellex is administered subcutaneously using a special syringe that comes with the drug. When administered, use a sterile filter attachment on the syringe. The required amount of solution is collected and administered using different needles. When using Cellex, local allergic reactions are possible, expressed in blood flow to the injection site, skin rash, a feeling of painful tickling irritation of the skin, causing the need to scratch the irritated area, angioedema, increased body temperature to subfebrile values, decreased quality of sleep, and headaches.
Cellex is not used for epilepsy, manic-depressive psychosis, delirium, delirium tremens, as well as during pregnancy and breastfeeding (due to lack of data). Persons with malignant decompensated arterial hypertension, panic attacks, and severe mixed anxiety-depressive disorders should take Cellex with extreme caution. The drug does not contain infectious agents whose reproduction occurs without the participation of nucleic acids. If the patient has a history of allergies to protein-based drugs, regular medical supervision should be provided. When Cellex is combined with drugs that stimulate the central nervous system and with ethanol-containing products, motor restlessness, speech agitation, and sleep disorders may occur. Cellex may reduce the effect of tranquilizers and antipsychotics. The effectiveness of the drug in cerebrovascular diseases has been clinically confirmed. In our country, such diseases are an acute problem, not only medical, but also social. Every year there is an increase of 450 thousand cases of strokes, half of which are fatal in the first year. Cellex helps prevent acute circulatory disorders in brain tissue, and also accelerates recovery time after ischemic episodes.