Cortisol (Hydrocortisone)
Steroid hormone of the adrenal cortex; the most active of the glucocorticoid hormones.
Regulator of carbohydrate, protein and fat metabolism. Cortisol is produced by the zona fasciculata of the adrenal cortex under the control of ACTH. In the blood, 75% of cortisol is bound to corticosteroid binding globulin (transcortin), which is synthesized by the liver. Another 10% is weakly bound to albumin. Cortisol is metabolized in the liver, the half-life of the hormone is 80-110 minutes, it is filtered in the glomeruli and eliminated in the urine.
This hormone plays a key role in the body's defense response to stress. It has a catabolic effect. Increases the concentration of glucose in the blood by increasing its synthesis and reducing utilization in the periphery (insulin antagonist). Reduces the formation and increases the breakdown of fats, promoting hyperlipidemia and hypercholesterolemia. Cortisol has little mineralocorticoid activity, but when it is formed in excess, sodium retention in the body, edema and hypokalemia are observed; a negative calcium balance is formed. Cortisol potentiates the vasoconstrictor effect of other hormones and increases diuresis. Cortisol has an anti-inflammatory effect and reduces the body's hypersensitivity to various agents, suppressing cellular and humoral immunity. Cortisol stabilizes lysosome membranes. Helps reduce the number of zosinophils and lymphocytes in the blood while simultaneously increasing neutrophils, erythrocytes and platelets.
The daily rhythm of secretion is characteristic: maximum in the morning (6-8 hours), minimum in the evening (20-21 hours). Cortisol secretion changes little with age. During pregnancy, a progressive increase in concentration is observed, associated with an increase in the content of transcortin: in late pregnancy, a 2-5-fold increase is noted. The daily rhythm of release of this hormone may be disrupted. In the case of a partial or complete block in the synthesis of cortisol, an increase in the concentration of ACTH and the total concentration of corticoids occurs.
Limits of determination:
27.6 nmol/l-6599.6 nmol/l.
Most common symptoms
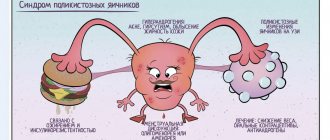
Regardless of the source of hyperandrogenism (that is, an increase in the amount of male sex hormones), clinical manifestations include:
- 1 Hirsutism is the appearance of hair in unwanted places (on the face, chest, arms, thighs, buttocks, near the nipples). The so-called shaft hair grows (dense, thick, brightly pigmented).
- 2 Disorders of the menstrual cycle according to the type of hypomenstrual syndrome: from oligomenorrhea (scanty, infrequent menstruation) to amenorrhea (absence of menstruation for a year or more).
- 3 Absence of pregnancy , and if it occurs, spontaneous termination in the early stages (frozen pregnancy is more often diagnosed).
- 4 Excessive hair growth on the legs , back, and forearms.
- 5 With a long course of the disease, virilization syndrome appears. In women it manifests itself as deepening of the voice, baldness, and enlargement of the clitoris.
The severity of the above symptoms depends on the level of androgens in the body and the duration of the disease.
Thyroid-stimulating hormone (TSH, thyrotropin)
A glycoprotein hormone that stimulates the formation and secretion of thyroid hormones.
It is produced by basophils of the anterior pituitary gland under the control of thyroid-stimulating hypothalamic releasing factor, as well as somatostatin, biogenic amines and thyroid hormones. Increases vascularization of the thyroid gland. Increases the supply of iodine from blood plasma to thyroid cells, stimulates the synthesis of thyroglobulin and the release of T3 and T4 from it, and also directly stimulates the synthesis of these hormones. Enhances lipolysis.
There is an inverse logarithmic relationship between the concentrations of free T4 and TSH in the blood.
TSH is characterized by daily fluctuations in secretion: blood TSH reaches its highest values at 2 - 4 am, the highest level in the blood is also determined at 6 - 8 am, the minimum TSH values occur at 17 - 18 pm. The normal rhythm of secretion is disrupted when awake at night. During pregnancy, the concentration of the hormone increases. With age, the concentration of TSH increases slightly, and the amount of hormone emissions at night decreases.
Limits of determination:
0.0025 mU/l-100 mU/l.
Preparing for tests
Two days before collecting biological material at OPG17, you should stop:
- consumption of alcoholic beverages;
- smoke;
- take anticoagulants;
- physical exercise;
- stressful situations.
Before visiting a hospital facility, the patient should skip breakfast (the last meal no later than 8 hours before the blood draw). Doctors allow you to drink, but only clean water and in limited quantities.
The patient must tell the laboratory technician what medications he is taking.
Follicle stimulating hormone (FSH)
Glycoprotein gonadotropic hormone of the pituitary gland. Stimulator of the development of seminiferous tubules and spermatogenesis in men and follicles in women.
It is synthesized by basophilic cells of the anterior pituitary gland under the control of gonadoliberin, sex hormones and inhibin. FSH is released into the blood in pulses at intervals of 1 to 4 hours. The concentration of the hormone during the release is 1.5 - 2.5 times higher than the average level; the release lasts about 15 minutes. There are seasonal fluctuations in the concentration of the hormone in the blood: in summer, the level of FSH in men is higher than at other times of the year.
In women, FSH stimulates the formation of follicles. Reaching a critical level of FSH leads to ovulation. In men during puberty, FSH triggers spermatogenesis and then participates in its maintenance. FSH is the main stimulator of seminiferous tubule growth. FSH increases the concentration of testosterone in plasma, thereby ensuring the process of sperm maturation.
The LH/FSH ratio is important. Normally before menarche it is 1; a year after menarche - from 1 to 1.5; in the period from two years after menarche to menopause - from 1.5 to 2.
Limits of determination:
0.05 mU/ml-750 mU/ml.
Due to the pulsatile nature of the release of FSH and LH, in conditions that lead to a decrease in the level of these hormones, it may be useful to study three consecutive blood samples, each 30 minutes apart. In conditions associated with elevated FSH levels (such as gonadal dysfunction during menopause), taking a single sample is adequate.
Luteinizing hormone (LH)
Glycoprotein gonadotropic hormone. It is synthesized by basophilic cells of the anterior pituitary gland under the influence of releasing factors of the hypothalamus.
In women, it stimulates the synthesis of estrogen; regulates the secretion of progesterone and the formation of the corpus luteum. Reaching a critical level of LH leads to ovulation and stimulates the synthesis of progesterone in the corpus luteum. In men, by stimulating the formation of sex hormone binding globulin (SHBG), it increases the permeability of the seminiferous tubules to testosterone. This increases the concentration of testosterone in the blood plasma, which promotes sperm maturation. In turn, testosterone re-inhibits the release of LH. In men, LH levels increase between 60 and 65 years of age.
The release of the hormone is pulsating in nature and depends in women on the phase of the ovulation cycle. During puberty, LH levels increase, approaching values typical for adults. In the menstrual cycle in women, the peak concentration of LH occurs at ovulation, after which the level of the hormone drops and remains throughout the luteal phase at lower values than in the follicular phase. During pregnancy the concentration decreases. During the postmenopausal period, the concentration of LH increases, as does FSH (follicle-stimulating hormone). In women, the concentration of LH in the blood is maximum in the period from 12 to 24 hours before ovulation and is maintained throughout the day, reaching a concentration 10 times higher compared to the non-ovulatory period.
The LH/FSH ratio is important. Normally before menarche it is 1; after a year of menarche - from 1 to 1.5; in the period from two years after menarche to menopause - from 1.5 to 2.
Limits of determination:
0.09 mU/ml-1000 mU/ml.
When control is needed
If a woman plans to become a mother and is undergoing infertility therapy, then she needs to monitor the level of the hormone. OPG17 is also detected at different stages of pregnancy, in the presence of chronic diseases of the adrenal glands.
Patients with the following pathological conditions can receive a referral to the laboratory:
- menstrual irregularities;
- the appearance of pimples, blackheads, and blackheads in adolescence;
- polycystic ovary syndrome;
- corpus luteum cysts;
- Itsenko-Cusheng disease, etc.
Prolactin
A polypeptide hormone that stimulates the proliferation of the mammary gland and milk secretion.
Prolactin
Produced in the anterior lobe of the pituitary gland, a small amount is synthesized in peripheral tissues. During pregnancy, it is also produced in the endometrium. During pregnancy, prolactin supports the existence of the corpus luteum and the production of progesterone, stimulates the growth and development of the mammary glands and milk production. This is one of the hormones that contribute to the formation of sexual behavior. Prolactin regulates water-salt metabolism, delaying the excretion of water and sodium by the kidneys, and stimulates the absorption of calcium. In general, prolactin activates anabolic processes in the body. Other effects include stimulation of hair growth. Prolactin also has a modulating effect on the immune system.
The daily secretion of prolactin has a pulsating character. During sleep, its level increases. After waking up, the concentration of prolactin decreases sharply, reaching a minimum in the late morning hours. After noon, the hormone level increases. In the absence of stress, daily fluctuations in levels are within normal values. During the menstrual cycle, prolactin levels are higher in the luteal phase than in the follicular phase. From the 8th week of pregnancy, prolactin levels increase, reaching a peak at 20 - 25 weeks, then decrease immediately before childbirth and increase again during lactation.
Test for the presence of macroprolactin
is carried out as an additional study to the determination of prolactin when an elevated prolactin level is detected (according to the relevant recommendations - for all patients with a prolactin result > 700 mU/l). Prolactin can be present in the blood in different molecular forms.
Macroprolactin
is prolactin, bound in immune complexes with antibodies, present in the blood in varying quantities. It is cleared from the blood more slowly than monomeric prolactin and can accumulate in high concentrations. This form of prolactin is less bioactive, and patients with high levels of macroprolactin may not have the classic symptoms associated with increased prolactin concentrations.
The results of this study should be taken into account when interpreting elevated prolactin values, discrepancies between study results and the overall clinical picture, and lack of reproducibility when conducting studies in different laboratories. Please note that performing a macroprolactin test does not increase the cost of prolactin determination. Detection of the possible significant presence of macroprolactin in samples from hyperprolactinemic patients is necessary to eliminate diagnostic errors, the need to prescribe unnecessary biochemical and radiological studies, and also to prevent inappropriate drug therapy or surgical intervention.
Limits of determination:
12.6 honey/l-172200 honey/l.
How to reduce
Before starting therapy, the cause of the pathological condition should be established. This may require additional clinical studies of progesterone, testosterone, and other hormone levels. They also conduct special stress tests to identify congenital adrenocortical insufficiency, ultrasound diagnostics, computer and magnetic resonance imaging. Once the final diagnosis is made, a treatment plan is developed.
Decreased progesterone in PCOS and tumors
Infertility due to PCOS requires complex therapy. For this purpose, combined oral contraceptives (COCs) are prescribed, which correct the menstrual cycle and hydroxyprogesterone levels. If pregnancy does not occur after correction, other methods of therapy are used - stimulation of ovulation or surgical treatment with dissection of the sclerotic membrane of the ovaries.
Treatment tactics for hormone-producing cysts, ovarian tumors or adrenal tumors are usually surgical. The pathological focus is removed surgically. For corpus luteum cysts, in some cases, conservative therapy with COCs is possible. Only after this do they plan to become pregnant.
Decreased hormone levels due to adrenal dysfunction
Congenital adrenal dysfunction is treated with glucocorticoids. The medicine, dosage and duration of administration are selected by the doctor.
Dexamethasone or Methylprednisolone tablets are usually prescribed. The dose of the drug is divided into 2-3 doses per day. Therapy is carried out until 17 OH progesterone decreases, then the dosage of the drug is gradually reduced and pregnancy is planned.
During gestation, the pregnant woman is under the supervision of a doctor. The decision to continue therapy during pregnancy is made individually in each case. If the level of hydroxyprogesterone remains high and exceeds the norm by 2-3 times, hormonal therapy is again prescribed, especially when the female gender of the fetus is confirmed. In severe cases, the drug may need to be taken throughout life.
Treatment for Itsenko-Cushing syndrome
It can also be treated either conservatively (hormonal therapy) or surgically. For example, for pituitary tumors, it is possible to irradiate the affected area or remove the tumor.
Since hormonal drugs are used to reduce the level of 17 OP, treatment must be prescribed and monitored by a doctor. It is impossible to correct hormonal levels by other means, but they can be used as an additional factor.
Nutrition for high progesterone
Traditional medicine uses infusions of rowan flowers, cloves (flowers, buds), carrot seeds, hogweed stem and others. But you can take them only after consulting a doctor.
Proper dietary nutrition can also speed up treatment. A diet for such a pathology should include a minimum amount of protein products and be rich in vegetables and fruits. Dishes made from carrots, potatoes, beets, onions, and legumes (peas) are healthy. It is also necessary to saturate the body with vitamin C, which is abundant in citrus fruits, fresh herbs, sea buckthorn berries, and raspberries.
An increase in hydroxyprogesterone levels indicates hormonal disorders that are associated with various pathologies that require treatment and correction. It can cause infertility and miscarriage. Treatment is selected depending on the cause that caused the hormonal changes.
Estradiol
The most active estrogenic (female) sex steroid hormone.
In women, it is produced in the ovaries, placenta and zona reticularis of the adrenal cortex under the influence of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and prolactin. Estradiol is formed in small quantities during the peripheral conversion of testosterone. In men, estradiol is formed in the testes, in the adrenal cortex, but most of it is formed in peripheral tissues due to the conversion of testosterone.
In women, estradiol ensures the formation of the reproductive system according to the female type, the development of female secondary sexual characteristics during puberty, the formation and regulation of menstrual function, the development of the egg, the growth and development of the uterus during pregnancy; is responsible for the psychophysiological characteristics of sexual behavior. Ensures the formation of subcutaneous fat tissue according to the female type. By reducing the resistance of the uterine vessels, it increases blood flow in it and stimulates endometrial hyperplasia. Ovulation occurs 24 to 36 hours after the occurrence of above-threshold estradiol levels. A necessary condition for the effects of estradiol to occur is the correct relationship with testosterone levels. Estradiol has an anabolic effect, enhances bone turnover and accelerates the maturation of skeletal bones. Promotes sodium and water retention in the body. Reduces cholesterol levels and increases blood clotting activity. Estradiol affects the release of neurotransmitters, contributing to increased nervous tension and irritability.
Daily fluctuations in the concentration of estradiol in the serum are associated with the rhythm of LH (luteinizing hormone) secretion: the maximum occurs from 15 to 18 hours, and the minimum between 24 and 2 hours. In men, the level of estradiol progressively increases, in boys the increase occurs to a lesser extent. In women of childbearing age, the level of estradiol in blood serum and plasma depends on the phase of the menstrual cycle. At the beginning of the cycle, the concentration of estradiol slowly increases. The highest levels of estradiol are observed in the late follicular phase. After ovulation, the hormone level decreases, and a second, smaller amplitude, rise occurs. Then there is a decline in the concentration of the hormone, which continues until the end of the luteal phase. During pregnancy, the concentration of estradiol in serum and plasma increases at the time of delivery, and after delivery it returns to normal on the 4th day. With age, women experience a decrease in estradiol concentrations. During postmenopause, the concentration of estradiol decreases to the level observed in men.
Limits of determination:
37.0 pmol/l-40370 pmol/l.
Dehydroepiandrosterone sulfate (DEA-S04)
Androgenic hormone of the adrenal glands.
Produced in the adrenal cortex. The level of this hormone is an adequate indicator of androgen-synthetic activity of the adrenal glands. The hormone has only a weak androgenic effect, however, during its metabolism, testosterone and dihydrotestosterone are formed in peripheral tissues. Does not show noticeable daily fluctuations and has a low clearance rate.
During pregnancy, it is produced by the adrenal cortex of the mother and fetus and serves as a precursor for the synthesis of placental estrogens. Its level increases during puberty, and then gradually decreases as a person reaches reproductive age. During pregnancy, the level of this hormone also decreases.
The determination of DHEA-SO4 replaces the determination of 17-CS in urine in assessing the production of adrenal androgens. DEA sulfate synthesis does not occur in the ovaries (therefore, the test is used to determine the source of hyperandrogenemia in a woman’s body).
Limits of determination:
0.08-81.42 µmol/l..
Testosterone
A steroidal androgenic hormone that determines the development of secondary sexual characteristics, puberty and normal sexual function.
In men, the main part is synthesized in the testicle; a smaller amount is produced by cells of the reticular layer of the adrenal cortex and during transformation from precursors in peripheral tissues. In women, testosterone is formed during the process of peripheral transformation, as well as during synthesis in the cells of the inner lining of the ovarian follicle and the reticular layer of the adrenal cortex.
Testosterone has anabolic effects on muscle tissue, promotes the maturation of bone tissue, stimulates the formation of sebum by the skin glands, is involved in the regulation of lipoprotein synthesis by the liver, and modulates the synthesis of b-endorphins (“joy hormones”) and insulin. In men, it ensures the formation of the reproductive system according to the male type, the development of male secondary sexual characteristics during puberty, activates sexual desire, spermatogenesis and potency, and is responsible for the psychophysiological characteristics of sexual behavior. In women, it is involved in the mechanism of follicle regression in the ovaries and in the regulation of the level of gonadotropic hormones of the pituitary gland.
In men, testosterone levels increase during puberty and remain high until, on average, age 60. The level of the hormone in the blood plasma fluctuates throughout the day. The maximum concentration is observed in the morning, the minimum in the evening. In autumn, testosterone concentrations increase. In women, the maximum concentration of testosterone is determined in the luteal phase and during ovulation. In pregnant women, testosterone concentration increases by the third trimester, exceeding almost 3 times the concentration in non-pregnant women. During menopause, testosterone concentrations decrease.
Limits of determination:
0.15 nmol/l-120 nmol/l.
Sex hormone binding globulin (SHBG)
A blood plasma protein involved in the binding and transport of sex hormones.
There are several synonyms for the name of this protein: sex steroid binding globulin, androgen binding globulin, sex steroid binding globulin, sex hormone-binding globulin. This glycoprotein is synthesized in the liver; its molecular weight is about 80,000 - 100,000 daltons, the molecule has 1 binding site for steroid hormones. SHBG binds testosterone and 5-dihydrotestosterone with high affinity and estradiol somewhat weaker.
Testosterone circulates predominantly as bound to SHBG, and to a lesser extent to albumin and cortisol-binding globulin. Because variations in carrier proteins can influence circulating testosterone concentrations, SHBG is usually determined in addition to total testosterone measurements. The level of SHBG synthesis in the liver depends on sex hormones: estrogens increase, and androgens reduce its production. Therefore, the SHBG content in women is almost twice as high as in men. With a decrease in estradiol production, the total content of the hormone and the concentration of free hormone in the blood decrease in parallel.
With a decrease in androgen production, an increase in SHBG production causes total testosterone to remain constant, although the concentration of free hormone decreases. Therefore, plasma total testosterone levels may be paradoxically normal in early stages of testicular disease. Reduced SHBG levels are often found in hirsutism, acne vulgaris and polycystic ovary syndrome. With hirsutism, a decrease in SHBG is described in approximately 30% of women examined.
SHBG levels may be significantly increased in late pregnancy or after estrogen administration. Androgen administration is often combined with decreased SHBG levels. The Free androgen index (FAI), calculated as the ratio of total testosterone to SHBG in %, correlates with biologically available free testosterone and is used as a useful indicator of pathological androgen status.
After age 60, SHBG levels increase by approximately 1.2% per year, so with age, the level of biologically available testosterone decreases to a greater extent than the level of total testosterone.
Progesterone and 17-OHP: what's the difference?
Quite often, 17-OH-progesterone and progesterone are confused. These substances are indeed in close relationship with each other: in the presence of certain enzymes, 17-OHP, its metabolite, is formed from progesterone.
In contrast, progesterone is an active hormone and has the following functions:
- 1 Triggers an enzyme cascade that results in ovulation in the ovaries.
- 2 Ensures the development of the endometrium in phase 2 and affects its duration.
- 3 Has a relaxing effect on the muscles of the uterus.
- 4 Blocks the effect of estrogen on the body.
- 5 Contributes to the preservation and gestation of pregnancy.