Hydatidiform mole (MH) is a fatal disease for the fetus concerning the pathological development of the fetal egg and associated with the transformation of the chorion into cysts (protective membranes formed due to unfavorable conditions in certain life cycles, in the form of bubbles containing fluid) and the proliferation of the villi epithelium outer germinal membrane.
This pathological condition is accompanied by the following negative manifestations:
- bleeding;
- early toxicosis;
- enlargement of the uterus relative to the gestational age of the fetus.
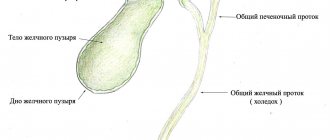
PZ is diagnosed using a vaginal examination, ultrasound, fetal phonocardiography, and determination of the content of human chorionic gonadotropin (β-hCG).
Treatment includes techniques such as vacuum aspiration, curettage of the uterine cavity, and removal of the organ (hysterectomy).
What is a hydatidiform mole?
Hydatidiform mole is a pathology that is characterized by modification of the membrane tissue (chorionic hyperplasia) into peculiar cysts. These are cavities, bubbles filled with liquid contents. Next, the growth of such chorionic villi occurs, which is accompanied by the death of the embryo developing in utero.
This pathology belongs to trophoblastic diseases. The incidence of such a diagnosis is about 0.5%. Characteristic features of the pathological condition are swelling and a sharp proliferation of villi tissue, the formation of bubble-like formations. In its form, this process resembles a bunch of grapes. The size of these bubbles is quite impressive. Can be about 2-2.5 centimeters in diameter. There is practically no blood flow in such bubbles.
Reasons for the development of the anomaly
There are several reasons for the formation of such a pathological condition as hydatidiform mole:
- Chromosomal abnormalities resulting from the prenatal period. With the complete variant of the pathology, there is a complete loss of maternal genetic material. The same situation can arise when two male reproductive cells fertilize an inferior egg without a nucleus.
- Viral or bacterial infection of the chorionic villi, leading to the above changes.
- Multiparous women, as well as young primigravidas, are at risk of developing the process.
- Immunodeficiency states.
- Thyrotoxicosis.
Types of hydatidiform mole
- Complete hydatidiform mole is a condition in which all chorionic villi are subject to pathological changes in the form of stromal edema, trophoblast hypertrophy and the formation of vesicles, while the signs of a developing embryo are completely absent. All tissue is affected. This pathology is most often detected at 10-12 weeks of gestation. Such a hydatidiform mole contains a diploid number of chromosomes: 46 XX. It is worth saying that all chromosomes are paternal. There may be a karyotype of 46 XY, however, both X and Y are also paternal. The danger of this pathology is that in twenty percent of cases this process can become malignant with the formation of many metastases.
- Partial hydatidiform mole is characterized by the presence of some intact tissue. Intact parts of the embryo and placenta may be identified. The chromosome set is defined as 69 XXX, 69 XXX. The set contains 1 maternal chromosome. The frequency of transition to a malignant form of the process is about five percent.
- Invasive hydatidiform mole. This pathology is a process in which chorion grows into the myometrium of the uterus. This is a severe pathology that can lead to massive bleeding during removal of hydatidiform mole tissue.
According to histological structure, pathology is also divided into:
- Syncytial type;
- Cytotrophoblastic;
- Mixed form of hydatidiform mole.
Publications in the media
Hydatidiform mole is a condition accompanied by the proliferation of trophoblast (the outer layer of embryonic cells that is involved in the implantation of the embryo into the uterine wall and the formation of the placenta), filling the uterine cavity. Hydatidiform mole can be complete (classic) or incomplete (partial). With a complete hydatidiform mole, the changes affect the entire chorion; with a partial mole, only part of it. In addition, there is a malignant form of hydatidiform mole - destructive hydatidiform mole.
Statistical data. In the USA, 1 case of hydatidiform mole occurs in 1200 pregnancies, in the countries of the Far East - 1 case in 120 pregnancies, in Russia - 1 case in 820-3000 births. The predominant age is up to 30 years. Gestational trophoblastic disease (including hydatidiform mole, malignant trophoblastic tumors, and placental site trophoblastic tumor) occurs more commonly in women of low socioeconomic status and in underdeveloped regions (eg, Southeast Asia).
Etiology • Complete hydatidiform mole occurs with uniparental disomy, when for unknown reasons the loss of maternal genes and duplication of the paternal haploid genome occurs (the zygote has a karyotype of 46,XX). Sometimes (5%) a complete hydatidiform mole is caused by the fertilization of an “empty” (nucleated) egg by two sperm, resulting in a karyotype of 46,XY or 46,XX. The embryo dies in the early stages of development, before placental circulation is established • Incomplete hydatidiform mole is caused by triploidy as a result of fertilization of the egg by two sperm (dispermia) with a delay in the haploid set of maternal chromosomes. Conceptus cells contain one haploid set of maternal chromosomes and a diploid set of paternal chromosomes - the karyotype can be 69,XXY, 69,XXX or 69,XYY. The fetus dies.
Pathomorphology • Complete, or classic, hydatidiform mole •• Severe edema and enlargement of villi with transparent contents •• Disappearance of the blood vessels of the villi •• Proliferation of the trophoblastic lining of the villi, much less often degeneration •• Absence of the fetus, umbilical cord or amniotic membrane •• Normal karyotype (usually XX, less often XY) • Incomplete, or partial, hydatidiform mole •• Severe swelling of the villi with atrophy of trophoblast cells •• Presence of normal villi •• Presence of the fetus, umbilical cord and amniotic membrane •• Pathological karyotype, usually triploidy or trisomy.
Clinical picture • Bleeding, usually occurring in the first trimester of pregnancy • The uterus is larger than expected, given the date of the last menstrual period, at this stage of pregnancy • Nausea and vomiting, occurring in about a third of patients • Signs of preeclampsia in the first trimester of pregnancy • No reliable signs pregnancy in the form of determining parts of the fetus, heartbeat, fetal movements, ultrasound reveals only small cystic tissue in the uterus in the absence of the fetus • Hyperthyroidism sometimes develops. It is assumed that when the level of hCG increases excessively, this hormone binds to TSH receptors, causing hyperfunction of the thyroid gland • Abdominal pain bothers 15% of patients. The cause of pain is the formation of thecal lutein cysts under the influence of HCG in 50% of patients.
Destructive form of hydatidiform mole • Hydatidiform mole tissue penetrates into the thickness of the uterine wall and metastasizes to the lungs, vagina, parametrial tissue • Clinical picture - continued bleeding from the uterus after removal of the hydatidiform mole; the uterus does not contract; pain persists in the lower abdomen, sacrum, and lower back; when it grows to the peritoneum - the picture of an “acute abdomen”; theca lutein cysts do not undergo reverse development, the level of hCG is high • Treatment - see Gestational trophoblastic disease.
Diagnostics • The main evidence of hydatidiform mole is the presence of many bubbles with transparent contents in the vaginal discharge • An increase in the level of hCG of more than 100,000 mIU/ml with uterine enlargement and bleeding • Ultrasound shows no signs of a normal ovum or fetus.
TNM classification - see Gestational trophoblastic disease.
TREATMENT
• Vacuum aspiration. To remove a hydatidiform mole, they use more often than other methods, even if the uterus is enlarged to a size corresponding to 20 weeks of pregnancy •• After vacuum aspiration, oxytocin is administered intravenously for better contraction of the myometrium •• With significant bleeding and a large size of the uterus (over 20 weeks of pregnancy) Laparotomy with hysterectomy may be performed.
• Primary hysterectomy. If a woman does not want to have children in the future, a hysterectomy can be performed. The ovaries are not removed. If multiple thecal lutein cysts are present in the ovaries, their reverse development occurs after a drop in the level of hCG.
• Preventive chemotherapy. Preventive chemotherapy is carried out after removal of a hydatidiform mole, if the hCG titer increases or remains at a constant level for a long time, as well as when metastases are detected. In 80% of patients with hydatidiform mole, spontaneous remission occurs without additional therapy. Systematic determination of hCG levels helps to promptly identify developing chorionepithelioma; Therefore, given the high likelihood of toxic effects, prophylactic chemotherapy is not given to all patients.
Observation. The time for complete elimination of hCG (average 73 days) depends on the initial concentration of hCG, the amount of viable trophoblast tissue remaining after vacuum aspiration, and the half-life of hCG. Monitoring patients after removal of a hydatidiform mole includes a number of measures: • Determination of the level of hCG with an interval of 1–2 weeks until 2 negative results are obtained. Then studies are carried out monthly for 2 years. Patients are recommended to protect themselves from pregnancy for 2 years with oral contraceptives that reduce LH levels • Physical examination of the pelvic organs every 2 weeks until remission, then every 3 months for 1 year • In the absence of a decrease in the HCG titer, a chest x-ray to rule out metastases to the lungs.
Complications • Development of malignant trophoblast tumors (destructive, or invasive, hydatidiform mole, choriocarcinoma) with or without metastases • Bleeding • DIC syndrome • Embolism of the branches of the pulmonary artery by trophoblast cells.
Forecast. In 20% of cases of complete hydatidiform mole, the development of a malignant tumor is subsequently observed.
Synonyms • Chorioadenoma • Persistent trophoblastic disease • Invasive mole.
ICD-10 • O01 Hydatidiform mole.
Symptoms
There are several signs for the clinical picture of hydatidiform mole:
- Dark bloody discharge from the genital tract, which contains bubbles rejected from the site of hydatidiform mole. The volume of such bleeding can vary significantly, ranging from spotting to massive bleeding.
- If the bleeding is large or prolonged, the woman may develop anemia. Its symptoms include weakness, fatigue, pale skin, rapid heartbeat, and sometimes episodes of loss of consciousness.
- In the invasive form of this pathology, when chorionic villi grow into the wall of the uterus (myometrium), pain in the lower abdomen may occur. When a perforation of the uterine wall occurs, massive bleeding into the abdominal cavity may occur, which is accompanied by acute abdominal pain and loss of consciousness.
- The size of the uterus is not commensurate with gestational age. When such a pathological condition develops, the uterus is larger than the period calculated from the first day of the last menstruation.
- Toxicosis during hydatidiform mole is often several times more pronounced. Symptoms quickly increase, nausea and vomiting are debilitating, and liver and kidney failure may occur. This quickly leads a woman to a moderate or even severe state.
Diagnostics
Gynecological examination.
- When examined in the doctor's chair, you should be alert to the uterus, which is revealed to be much larger in size than the expected gestational age based on the date of the last menstruation.
- Dense consistency of the uterus with areas of strong softening.
- Bloody discharge from the genital tract with blisters.
Ultrasound examination, which is the most informative method for diagnosing this pathological condition. Ultrasound signs of hydatidiform mole include:
- Increased size of the uterus, which does not correspond to the timing of menstruation;
- The presence of a fine-grained tissue structure in the uterine cavity, reminiscent of a “blizzard”. This is a characteristic sign of a hydatidiform mole. This ultrasound picture is also sometimes called a “bunch of grapes”.
- A frequent occurrence during ultrasound diagnosis of the uterine appendages with such pathology is the presence of thecal lutein cysts.
- Blood sampling to determine hCG levels. Indeed, with hydatidiform mole, its level may exceed the borderline norms.
- If there is a suspicion of metastasis of a hydatidiform mole, a computed tomography or MRI is prescribed.
Diagnosis and staging
Diagnostics
Once a patient is diagnosed with elevated or rising hCG levels, careful laboratory testing of a complete peripheral blood count, biochemical analysis to assess liver and kidney function, and the initial hCG level are necessary. A gynecological examination is required to identify metastases in the vagina.
Vaginal metastases can cause heavy bleeding. Instrumental examination should include ultrasound of the pelvic organs to assess the presence of trophoblastic tissue in the uterus, as well as the extent of the tumor in the pelvis.
Examination of the chest organs is mandatory, since the lungs are the site of the most common metastasis. Pulmonary metastases on computed tomography (CT) can be detected in 40% of patients with a negative conventional chest radiography (CHX).
However, CT of the chest is not a mandatory diagnostic procedure if lung metastases are detected by conventional radiography, and CT will not change the treatment strategy. In the absence of lung and vaginal metastases, metastases to the liver, brain and other organs are rare, and brain imaging is not performed, which is an error.
Magnetic resonance imaging (MRI) with contrast should be performed in all women with metastatic or pathologically confirmed choriocarcinoma. However, there is no special need to histologically verify the diagnosis, since there is a high probability of developing difficult-to-control bleeding.
The role of positron emission computed tomography is to detect active tumor foci and confirm the activity of foci identified by other research methods.
Staging
FIGO and TM classification
The FIGO and TM classifications are used to classify trophoblastic tumors of pregnancy. The clinical classification of malignant trophoblastic tumor of pregnancy according to the TM system and FIGO stages is identical.
Unlike other localizations, category N (regional lymph nodes) is not used in the classification of this pathology. Histological confirmation is not required if hCG levels are abnormally elevated.
Medical history should be recorded prior to initiation of chemotherapy. The specificity of the classification of this tumor lies in the analysis of the anatomical distribution of the tumor, taking into account the main prognostic factors for the outcome of the disease.
The methods for assessing categories T and M are:
- Category T - Clinical examination, imaging techniques, endoscopic examination, serum and urine hCG levels;
- Category M - Clinical examination, imaging methods and assessment of HCG levels in serum and urine;
- Risk categories - Age, previous pregnancy outcome, time since diagnosis of pregnancy, serum/urinary HCG level before treatment, size of largest tumor, location of metastases, number of metastases, and the results of previous ineffective chemotherapy are also taken into account to calculate a prognostic indicator subdividing cases into high and low risk categories;
- T - Primary tumor;
- TX—Primary tumor cannot be assessed;
- T0 - The primary tumor is not determined;
- T1 - I Tumor limited to the uterus;
- T2 - II The tumor spreads to other genital structures: vagina, ovaries, broad ligament of the uterus, fallopian tube by metastasis or direct spread;
- M1a - III Metastases in the lung (them);
- M1b - IV Other distant metastases.
Note: *stages I-IV are divided into A and B in accordance with the prognostic indicator.
- M - distant metastases;
- M0 - no distant metastases;
- M1 - distant metastases;
- M1a - metastases in the lungs;
- M1b - metastases in other organs.
Note: Metastases in the genitals (vagina, ovaries, broad ligament of the uterus, fallopian tube) are classified as T2. Any lesion of non-genital structures (direct invasion or metastasis) is classified as M.
Treatment
The principles of treatment for hydatidiform mole include immediate removal of the contents of the uterus. In cases where a woman does not want to preserve her fertility, a hysterectomy may be performed.
A thorough histological examination of the removed material is necessary.
After removal of a hydatidiform mole, patients should be subjected to dynamic monitoring of the level of hCG in the blood serum. It is carried out weekly until its level becomes normal in three consecutive studies. This usually happens within 10 weeks after evacuation of the hydatidiform mole.
Subsequently, the level of hCG in the blood serum is monitored monthly for 6 months and then for another 6 months at two-month intervals.
Chemotherapy is a highly effective treatment for the vast majority of women with FNA.
Cure ranges from 100% at low risk to 80-90% at high risk in some centers. Despite successful treatment with chemotherapy, the role of other methods such as surgery and radiation therapy should not be overlooked. It is believed that the best treatment outcome is achieved when patients are treated under the auspices of a multidisciplinary team.
Low risk
Methotrexate and dactinomycin monochemotherapy are most widely used for low-risk tumors.
Monotherapy regimens:
- methotrexate 0.4 mg/kg intravenously or intramuscularly for 5 days, courses are repeated every 2 weeks (the next one starts on the 15th day from the start of the previous one);
- dactinomycin 12 mcg/kg intravenously for 10-15 minutes for 5 days, courses are repeated every 2 weeks (the next one starts on the 15th day from the start of the previous one);
- dactinomycin 1.25 mg/m2 intravenously for 10-15 minutes once every 2 weeks;
- methotrexate 1 mg/kg intravenously or intramuscularly on days 1, 3, 5 and 7;
- calcium folinate 0.1 mg/kg intravenously in a stream on days 2, 4, 6, 8, courses are repeated every 2 weeks (the next one starts on the 15th day from the start of the previous one).
Treatment is carried out until the hCG level is normalized, plus three courses of chemotherapy against the background of a normal marker. In cases of resistance to one of the drugs, monotherapy with the second is justified - in cases where, according to the total score, the patient remains in the low-risk group. In other cases, they switch to combination chemotherapy.
High risk
When treating high-risk patients, combination chemotherapy is indicated. Currently, the regimen of choice is the EMA-CO combination, with a cure rate of 70% to 90%. A similar EMA-EP regimen containing cisplatin can be used in cases of development of resistance to EMA-CO.
EMA-CO scheme:
- Day 1: etoposide 100 mg/m2 intravenously over 1 hour, dactinomycin 0.5 mg intravenously over 10-15 minutes, methotrexate 100 mg/m2 intravenous bolus and then 200 mg/m2 as a continuous infusion over 12 hours ;
- Day 2: etoposide 100 mg/m2 intravenously over 1 hour, dactinomycin 0.5 mg intravenously over 10-15 minutes, calcium folinate 15 mg intravenously or orally every 12 hours 4 times, starting 24 hours after the start of methotrexate administration ;
- Day 8: vincristine 1 mg/m2 intravenous bolus,
- cyclophosphamide 600 mg/m2 intravenously over 20-30 minutes.
Courses are repeated every 2 weeks (the next one starts on the 15th day from the start of the previous one). Treatment is carried out until the hCG level is normalized, plus three courses of chemotherapy against the background of a normal marker.
In cases of tumor resistance to standard first-line treatment, cisplatin-containing chemotherapy regimens are used.
In cases where there is massive lung damage with corresponding clinical symptoms, it may be advisable to reduce the dose of cytostatics (sometimes up to 50%) in order to avoid pulmonary-cardiac decompensation.
EMA-EP scheme:
- etoposide 100 mg/m2 intravenous infusion over 1 hour on day 1;
- cisplatin 80 mg/m2 intravenous infusion at a rate of no more than 1 mg/min with pre- and post-hydration on day 1;
- from day 8, drugs are administered according to the EMA regimen (excluding the introduction of dactinomycin and etoposide on the second day); Day 8: etoposide 100 mg/m2 intravenous infusion over 1 hour, dactinomycin 0.5 mg intravenously over 10-15 minutes;
- methotrexate 100 mg/m2 intravenous bolus and then 200 mg/m2 as a continuous intravenous infusion for 12 hours;
- Day 9: calcium folinate 15 mg intravenous bolus or orally every 12 hours 4 times, starting 24 hours after the start of methotrexate administration.
Courses are repeated every 2 weeks (the next one starts on the 15th day from the start of the previous one).
PVB circuit:
- cisplatin 20 mg/m2 intravenous infusion at a rate of no more than 1 mg/min with pre- and posthydration on days 1-5 or 100 mg/m2 intravenous infusion at a rate of no more than 1 mg/min with pre- and posthydration on day 1 ;
- vinblastine 0.2 – 0.3 mg/kg intravenously as a bolus on days 1 and 2;
- bleomycin 30 mg/m2 intravenously for 10 minutes once a week (days 1, 8, 15).
Courses are repeated every 3 weeks (the next one starts on the 22nd day from the start of the previous one). In some cases, the localization of metastases may require adjustments to standard treatment tactics. For example, metastatic brain damage requires an increase in the dose of methotrexate to 1000 mg/m2 in the EMA-CO regimen. Administration of methotrexate at such a high dose requires alkalinization of the urine.
Depending on the size and number of metastases in the brain, radiation therapy can be applied to the entire brain at a dose of 40 g.
In case of metastatic liver damage, radiation therapy to the liver can be performed at a dose of 20 g. or intra-arterial chemotherapy.
It should be understood that radiation therapy to the brain or liver is more likely to prevent life-threatening bleeding than to cure metastatic disease.
In cases where conservative treatment does not lead to regression of the tumor (including metastases in the lungs, liver or brain), the possibility of surgical removal of the residual tumor should be considered. It is possible to perform embolization of liver metastases. Metastases in the spleen due to the high risk of bleeding from the splenic vessels are an absolute indication for splenectomy. Surgical treatment including hysterectomy is also indicated in cases of bleeding that cannot be stopped by conservative methods, as well as in cases of uterine perforation.
Trophoblastic tumor of the placental bed
The main method of treatment for patients with TOPL is surgical, since the tumor is little sensitive to chemotherapy and there is deep damage to the myometrium. During surgery, it is recommended to perform total or selective pelvic lymphadenectomy, since PLPL is characterized by a lymphogenous route of metastasis.
Possibility of pregnancy after treatment
Patients who successfully complete chemotherapy maintain normal reproductive function.
A total of 2657 pregnancies following chemotherapy have been reported in the literature. Of these, 77% of women had full-term babies, 5% had premature births, 1% of patients had stillbirths, and 15% had spontaneous miscarriages.
Despite the use of potentially teratogenic drugs, there have been no reports of malformations following chemotherapy. There is no difference in the timing of conception and pregnancy depending on the chemotherapy regimen.
Psychosocial problems
Women who develop FNA may experience significant mood disturbances, family problems, and anxiety about future pregnancies. Patients may experience clinically significant levels of anxiety, fatigue, anger, confusion, sexual problems, worry about future pregnancies, and worries about past pregnancies for long periods of time. Patients with metastases are at risk of mental disorders.
Treatment
The treatment tactics for hydatidiform mole are based on the evacuation of pathological tissue from the uterus (removal of hydatidiform mole). This can be done using the method of vacuum aspiration of the contents of the female reproductive organ. And then curettage of the uterine cavity is performed. If there is a condition that threatens the patient's life, for example, bleeding from an invasive mole, then doctors may decide to remove the uterus.
After evacuation of the contents from the uterine cavity, mandatory monitoring of the level of hCG in the blood is carried out weekly. If the hCG level does not fall over time, then chemotherapy treatment is necessary. Once every two weeks, ultrasound diagnostics of the uterine cavity and appendages is performed.
If no signs of chorionepithelioma are found, then chemotherapy is not performed, and the woman is prescribed clinical observation for a period of two years.
Gestational trophoblastic disease
Gestational trophoblastic disease (GTD) is a pathology that includes hydatidiform mole (complete and partial), invasive hydatidiform mole, choriocarcinoma, trophoblastic tumor of the placental bed and epithelioid trophoblastic tumor. These diseases are not very common, but they pose a great threat to the health and life of patients. In the recent past, the mortality rate from various forms of GTB could reach almost 100% (for example, in cases of metastatic choriocarcinoma) [1]. And, despite the successes achieved in the treatment of this disease, it is necessary to be wary of GTB.
Partial and complete hydatidiform mole
Presumably, this pathology was described back in 400 BC. Hippocrates. But it was only in 1895 that hydatidiform mole was associated with pregnancy. We now consider hydatidiform mole as a pregnancy anomaly in which trophoblast (both cyto- and syncytiotrophoblast) proliferation occurs; chorionic villi grow in the form of “bubbles”, and all this is combined with the absence of an embryo/fetus. There are two forms of hydatidiform mole: complete and partial [2].
A complete hydatidiform mole is characterized by the impossibility of identifying the embryo; trophoblast hyperplasia with varying levels of atypia is observed, the villi are devoid of blood vessels. The formation of “bubbles” is generalized. In almost 90% of cases, the abnormal tissue has the 46XX chromosome set, obtained by duplicating the DNA of a haploid sperm. The genetic apparatus of the egg is defective or inactive or completely absent. Thus, all genetic material comes from the father (except for mitochondrial DNA, of course). In other cases (≈ 10%), the cells have a set of 46XY or 46XX, which is formed by the fusion of two sperm [1], [3].
Partial hydatidiform mole is a pathology of a slightly different kind. In this case, it is possible to detect some embryonic tissue; chorionic villi are swollen and vary in size and shape. The villi have a scalloped shape, protruding “inclusions” are formed from the trophoblast, and blood flow is maintained in the villi. One of the main differences between a partial hydatidiform mole and a complete one: hyperplasia and the formation of “bubbles” are focal in nature, and atypia is extremely weakly expressed. In this case, the chromosome set is triploid, often 69XXY, which is formed by the fusion of an almost normal egg and two sperm [1], [3].
It is important to note: after a complete mole, trophoblastic neoplasia develops in 15-20% of cases (invasive mole or choriocarcinoma, see below), while with partial mole, neoplasia develops in less than 5% of cases [1]. .
Figure 1. Complete hydatidiform mole
The clinical picture of a complete hydatidiform mole is nonspecific; symptoms can vary quite widely among different patients. Among the most common manifestations are excessive uterine enlargement, anemia, toxemia, hyperthyroidism and respiratory failure. Thanks to ultrasound and hCG analysis, diagnosis of complete hydatidiform mole has become possible already in the first trimester, usually long before the appearance of the first symptoms.
The clinic of partial hydatidiform mole “mimics” the picture of a non-developing pregnancy. Uterine bleeding may be observed, while the size of the uterus is within normal limits [3].
Diagnosis is carried out mainly using ultrasound and hCG analysis. With a complete hydatidiform mole, generalized hyperplasia is clearly visible, and an increase in hCG levels helps to distinguish a hydatidiform mole from a frozen pregnancy. In the case of partial hydatidiform mole, the most specific will be focal cystic changes in the placenta, as well as an increase in the gestational sac by one and a half times (this may indicate triploidy). The combination of these two features gives a fairly high accuracy of the result.
The level of human chorionic gonadotropin naturally increases with trophoblast hyperplasia. In the case of complete hydatidiform mole, the hCG level can reach 100,000 mU/ml or higher; with partial, such figures are less common, but the increase, nevertheless, is also clearly defined [3].
It should be noted that only a pathologist can finally diagnose a complete or partial hydatidiform mole after histological verification.
Treatment for complete and partial hydatidiform moles involves removing abnormal tissue from the uterus; the most optimal method is vacuum aspiration. Women who are no longer planning a pregnancy may be advised to have a hysterectomy (this is especially justified in the case of a complete pregnancy) [3].
However, hydatidiform mole itself does not pose such a danger as possible neoplastic processes that often develop subsequently. As we remember, after a complete hydatidiform mole (and sometimes after a partial one), choriocarcinoma, invasive hydatidiform mole and other pathological conditions can develop, which are combined into the group of gestational trophoblastic neoplasia (GTN).
Chemotherapy was previously even recommended to prevent GTN. However, this approach is now considered unjustified [4]. The evidence base for preventive antitumor therapy is quite low, and the hypothetical benefit in preventing GTN remains only hypothetical, while the harm is quite real. Therefore, patients diagnosed with hydatidiform mole require systematic observation and constant monitoring of hCG levels (its sharp increase, or “plateau” (constantly high level of the hormone) may indicate the development of complications in the form of gestational trophoblastic neoplasia).
It should be noted that future pregnancy is not contraindicated: a woman can conceive and bear a healthy child. However, we must remember that the risk of recurrent hydatidiform mole increases. The probability of a second hydatidiform mole is ≈ 1%, a third is ≈ 15–18% [3].
Gestational trophoblastic neoplasia
Gestational trophoblastic neoplasia (GTN) is a term that combines four pathological conditions: invasive hydatidiform mole, choriocarcinoma, trophoblastic tumor of the placental bed, and epithelioid trophoblastic tumor. GTN is a serious condition that requires treatment with chemotherapy. But before considering neoplasia in more detail, it should be noted that thanks to modern methods - hCG monitoring, systematic observation and chemotherapy - more than 90% of cases end favorably [5].
About 50% of GTN are a consequence of complete or partial hydatidiform mole, 25% of them develop after ectopic pregnancy and another 25% due to premature birth.
The clinical picture varies, the most common symptoms being bleeding, uterine enlargement, and thecal lutein cysts. But in half of the cases the disease may not have any symptoms. The main diagnostic criterion for GTN is an increase in hCG levels [6].
The International Federation of Gynecology and Obstetrics (FIGO) gives the following stages of GTN [7]: stage 1: the disease develops within the uterus; Stage 2: neoplasia has spread beyond the uterus, but is limited to other genital structures (vagina, broad ligament); Stage 3: GTN metastasizes to the lungs; Stage 4: metastasis to other organs.
Treatment for any GTN is chemotherapy; Often one drug is enough; at stage 4, a combination of therapeutic agents is recommended. The most commonly used drugs are methotrexate and dactinomycin [6]. In case of drug tolerance or disease relapse, paclitaxel may be recommended [8].
Also, for GTN with a high risk of metastasis, the EMA/CO combination is used: etoposide, methotrexate, actinomycin-D (dactinomycin), cyclophosphamide and vincristine [9].
Invasive hydatidiform mole
Invasive is a hydatidiform mole that penetrates the myometrium by direct “expansion” into the tissue or through the veins. Approximately 10-17% of hydatidiform moles will be invasive, and of these, about 15% will metastasize (most often to the lungs or vagina) [1]. .
Figure 2. Invasive hydatidiform mole
Invasive hydatidiform mole is often diagnosed clinically, since invasion into the myometrium is accompanied by bleeding and pain, and measuring the level of hCG allows us to verify the correctness of the diagnosis. Therefore, therapy for invasive mole begins even without histological verification [1].
It should be noted that this pathology can manifest itself in an extremely unusual way. There are known cases of sudden death of a primiparous woman [10], spontaneous renal hemorrhage [11], the development of invasive mole with metastases due to iatrogenic perforation of the uterus [12], etc.
Choriocarcinoma
Approximately 2-3% of hydatidiform moles transform into choriocarcinoma, a malignant tumor characterized by direct invasion of the myometrium and abnormal trophoblastic hyperplasia and anaplasia. There are no chorionic villi, there is extensive necrosis in the tissue; Bleeding is common [1]. .
Figure 3. Choriocarcinoma
Choriocarcinoma also has many forms, and perhaps deserves a separate discussion. It should only be noted that this tumor metastasizes to almost any organ and any tissue [13].
Trophoblastic tumor of the placental bed and epithelioid trophoblastic tumor
Trophoblastic tumor of the placental bed (TPT) is an extremely rare malignant disease. It occurs when, at the site of implantation, trophoblast cells begin to divide and penetrate into the myometrium between its muscle fibers [1]. .
Figure 4. Tumor of the placental bed. Tumor cells penetrate into the tissue between the muscle fibers of the myometrium [14].
Typically, such a tumor has the form of a polypoid or nodular dense formation of small size (≈ 5 cm). The tumor itself is homogeneous, consisting of polygonal cells with pronounced atypia; often penetrates deep into the tissue [14], [15]. Oddly enough, TOPL responds extremely well to chemotherapy. If the patient does not have metastases, surgical treatment is recommended.
Epithelioid trophoblastic tumor (ET) is an even rarer form of TPLT that is somewhat similar to choriocarcinoma. It is a set of proliferating mononuclear trophoblast cells. In some cases, the tumor can partially replace the epithelium of the endocervix.
There is no significant difference in the treatment of TPL and ET, however it is important to note that epithelioid trophoblastic tumor can occur years after pregnancy [1], [16].
Sources:
- JR Lurain, 'Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole', Am. J. Obstet. Gynecol., vol. 203, no. 6, pp. 531–539, 2010.
- M. J. Seckl, N. J. Sebire, and R. S. Berkowitz, 'Gestational trophoblastic disease', Lancet, vol. 6736, no. 10, pp. 1–13, 2010.
- E. R. M. Jauniaux and J. Johns, 'Molar pregnancy', Early Pregnancy, pp. 67–74, 2010.
- Q. Wang et al., 'Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia', Cochrane Database Syst. Rev., vol. 2021, no. 9, 2021.
- JR Lurain, 'Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia', Am. J. Obstet. Gynecol., vol. 204, no. 1, pp. 11–18, 2011.
- A. Biscaro, A. Braga, and R. S. Berkowitz, 'Diagnosis, classification and treatment of gestational trophoblastic neoplasia', Rev. Bras. Ginecol. e Obs., vol. 37, no. 1, pp. 42–51, 2015.
- H. Y. S. Ngan, H. Bender, J. L. Benedet, H. Jones, G. C. Montruccoli, and S. Pecorelli, 'Gestational trophoblastic neoplasia, FIGO 2000 staging and classification', Int. J. Gynecol. Obstet., vol. 83, no. SUPPL. 1, pp. 175–177, 2003.
- J. M. Morgan and J. R. Lurain, 'Gestational trophoblastic neoplasia: An update', Curr. Oncol. Rep., vol. 10, no. 6, pp. 497–504, 2008.
- L. Deng, J. Zhang, T. Wu, and T. A. Lawrie, 'Combination chemotherapy for primary treatment of high-risk gestational trophoblastic tumor', Cochrane Database Syst. Rev., vol. 2013, no. 1, pp. 1–3, 2013.
- M. Chauhan, C. Behera, S. Madireddi, S. Mandal, and S. K. Khanna, 'Sudden death due to an invasive mole in a young primigravida: Precipitous presentation masquerading the natural manner', Med. Sci. Law, vol. 58, no. 3, pp. 189–193, 2021.
- S. Xiao, Q. Mu, Y. Wan, and M. Xue, Spontaneous renal hemorrhage caused by invasive mole: a case report, vol. 37, no. 3. 2016.
- Y. Shen, X. Wan, and X. Xie, 'A metastatic invasive mole arising from iatrogenic uterus perforation', BMC Cancer, vol. 17, no. 1, pp. 2–5, 2021.
- L. Jiao, E. Ghorani, N. J. Sebire, and M. J. Seckl, 'Intraplacental choriocarcinoma: Systematic review and management guidance', Gynecol. Oncol., vol. 141, no. 3, pp. 624–631, 2016.
- R. N. Baergen, J. L. Rutgers, R. H. Young, K. Osann, and R. E. Scully, 'Placental site trophoblastic tumor: A study of 55 cases and review of the literature emphasizing factors of prognostic significance', Gynecol. Oncol., vol. 100, no. 3, pp. 511–520, 2006.
- C. M. Feltmate, D. R. Genest, L. Wise, M. R. Bernstein, D. P. Goldstein, and R. S. Berkowitz, 'Placental site trophoblastic tumor: A 17-year experience at the New England Trophoblastic Disease Center', Gynecol. Oncol., vol. 82, no. 3, pp. 415–419, 2001.
- O. Fadare, V. Parkash, M. L. Carcangiu, and P. Hui, 'Epithelioid trophoblastic tumor: Clinicopathological features with an emphasis on uterine cervical involvement', Mod. Pathol., vol. 19, no. 1, pp. 75–82, 2006.
New pregnancy after hydatidiform mole
The most pressing question after a case of hydatidiform mole is whether pregnancy can occur after a hydatidiform mole.
The diagnosis of hydatidiform mole is not a death sentence. With proper and timely diagnosis and proper treatment, pregnancy is possible. An important point is the timely administration of chemotherapy after a hydatidiform mole in the absence of a drop in hCG levels.
Pregnancy after a hydatidiform mole simply needs to be planned. If chemotherapy was not prescribed after such a pathological condition, then the minimum period of absence of pregnancy is 6-12 months. If chemotherapy was nevertheless prescribed, then the recommended break is 12-24 months. The main point in planning such a pregnancy is the appointment of competent contraception by the obstetrician-gynecologist who is caring for you.
Indications for treatment
After pregnancy with hydatidiform mole
Early diagnosis of pregnancy with hydatidiform mole using ultrasound (ultrasound) makes it possible to diagnose it in the early stages of its appearance.
After evacuation of a hydatidiform mole, the diagnosis of TOB is established based on the following data as defined by the International Federation of Gynecologists and Obstetricians (FIGO):
- elevated hCG levels in the form of a plateau for at least three weeks without a tendency to decrease;
- an increase in hCG of 10% or more with three or more measurements within two weeks;
- elevated hCG levels that persist for 6 months after evacuation of the hydatidiform mole;
- histological diagnosis of choriocarcinoma.
After pregnancy without hydatidiform mole
After a normal pregnancy, hCG levels are usually not determined. However, any woman of reproductive age with a history of pregnancy, abnormal bleeding, or known metastases should undergo hCG screening.
Careful clinical, instrumental and radiological studies should be performed to determine the stage of the disease with elevated hCG levels. Rapid growth, wide dissemination, and high tendency to bleed make this tumor a medical emergency.
Determination of hCG level
Normally, hCG is synthesized by syncytiotrophoblasts of the developing placenta. In contrast, hyperglycosylated hCG is synthesized by tumor trophoblasts. This glycoprotein consists of two subunits, the O±-subunit, common to glycoproteins of this group, and the OI-subunit, specific only to this hormone.
The level and nature of changes in OI-hCG is important for the diagnosis and assessment of the effectiveness of treatment for TAB. After evacuation of the hydatidiform mole, OI-hCG disappears after 8-10 weeks. Persistence of hCG levels indicates local or metastatic spread of the disease.
By monitoring serum or urine hCG levels, invasive disease can be diagnosed at an early stage and treatment can be initiated promptly. During treatment, the dynamics of changes in hCG levels should be carried out weekly in the same laboratory.
A change in hCG content during treatment is a guideline for the advisability of continuing treatment according to the current regimen or switching to another.
False positive hCG level
False increases in hCG may occur due to the presence of heterophilic antibodies that interfere with a good immunoassay. These conditions are extremely rare, but a false-positive hCG level can be misleading when trying to identify pregnancy disorders such as an ectopic pregnancy or TB.
Misinterpretation of a false-positive test may result in inappropriate treatment strategies, including surgery and chemotherapy, if based solely on elevated hCG levels.
A false-positive hCG result should be suspected in cases where the clinical picture does not correspond to laboratory data, if there is no history of pregnancy, or if patients are being treated with persistently low hCG levels that do not respond appropriately to treatment.
In rare cases, the source of the increase in hCG, especially in women approaching menopause, is the pituitary gland. If a false positive level of hCG is suspected, it is necessary to determine the content of this hormone in the urine, since heterophilic antibodies do not pass through the renal filter.