Introduction
Sodium-glucose cotransporter type 2 (SGT2) inhibitors have demonstrated significant cardiovascular benefits in patients with type 2 diabetes mellitus (T2DM) both with established cardiovascular disease and with multiple risk factors for it [1–3].
Several theories have been put forward to explain the beneficial effects of INGT2 on cardiovascular outcomes. Thus, studies have obtained data on a decrease in preload on the heart due to increased natriuresis, improvement in the metabolism of cardiomyocytes, and a decrease in the severity of fibrotic and inflammatory processes in the myocardium and vascular wall [4–6]. However, this topic continues to be the subject of active study. Type 2 diabetes mellitus is associated with the development of chronic inflammation due to various mechanisms. It has been shown that INGT2 can reduce the levels of some cytokines, such as tumor necrosis factor α, interleukin-6, high-sensitivity C-reactive protein (hsCRP) [7]. In addition, it is well known that fibrotic processes in the myocardium develop in patients with T2DM, regardless of the presence of arterial hypertension and the severity of coronary atherosclerosis [8]. In this regard, studying the processes of fibrosis and inflammation in patients with T2DM is relevant.
Purpose of the study:
to evaluate the dynamics of the level of serum markers associated with the processes of fibrosis and inflammation in T2DM during treatment with dapagliflozin.
Relevance
Patients with chronic kidney disease are at high risk of developing adverse events from the kidneys, blood vessels and heart.
Until now, the effect of dapagliflozin in patients with chronic kidney disease with or without diabetes mellitus was not known.
Material and methods
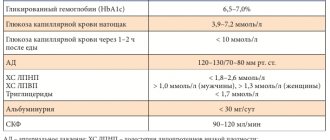
A single-center prospective study was conducted, approved by the ethics committee of the Federal State Budgetary Institution “National Medical Research Center named after. V.A. Almazov" of the Russian Ministry of Health. Before entering the study, all patients signed informed consent. Patients were included with T2DM lasting more than 1 year, glycated hemoglobin level from 7% to 10%, without verified atherosclerotic heart and vascular disease, but with multiple risk factors for cardiovascular events (dyslipidemia, obesity, arterial hypertension). In addition, inclusion criteria were age from 40 to 65 years, stable hypoglycemic, lipid-lowering and antihypertensive therapy for at least 12 weeks. before inclusion in the study. The study did not include patients with atrial fibrillation, valvular heart disease, rheumatological diseases, exacerbation of chronic diseases, type 1 diabetes mellitus, symptoms of hypotension, and systolic blood pressure (SBP) levels below 95 mmHg. Art., the level of N-terminal propeptide of natriuretic hormone (NT-proBNP) >125 ng/ml, glomerular filtration rate (GFR) <60 ml/min/1.73 m2 and chronic kidney disease, liver disease and also receiving insulin therapy, therapy glucocorticosteroids, mineralocorticoid receptor antagonists. Patients received dapagliflozin at a dose of 10 mg/day for 6 months. in addition to basic therapy for T2DM.
During the study, blood samples were collected before starting treatment with dapagliflozin and after 6 months. treatment. Blood samples were taken on an empty stomach (after 8 hours of fasting), and the following parameters were assessed: glycated hemoglobin (HbA1c), alanine aminotransferase, aspartate aminotransferase, creatinine, lipid profile (total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides), NT -proBNP, hsCRP, galectin-3, tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), matrix metalloproteinase-9 (MMP-9), stimulating growth factor expressed by gene 2 (ST2), carboxy-terminal fragment of type I procollagen (PIСP) . The hsCRP level was determined on a Cobas Integra 400+ analyzer using the immunoturbidimetric method. The concentration of NT-proBNP was determined by the electrochemiluminescent method using the Elecsys test system (Roche Diagnostic). Serum levels of galectin-3 (R&D system), MMP-9 and TIMP-1 (R&D system), sST2 (Clinical diagnostics, Presage ST2 kit), PICP (USCN Life Science) were assessed by enzyme immunoassay. In addition, body weight, height, waist circumference, and blood pressure (BP) were assessed at the beginning and end of the study. In order to assess the normal values of fibrosis biomarkers, the study additionally included 15 patients of a similar age without T2DM and other chronic diseases, including cardiovascular and kidney diseases (control group).
The SPSS Statistics program was used for statistical analysis. Quantitative data are presented as median and 25th and 75th quartiles - Me [Q25; Q75], qualitative characteristics - in the form of absolute and relative (%) indicators. Differences between quantitative indicators were identified using the Wilcoxon test. Correlation analysis was performed using Spearman's rank correlation coefficient. The null hypothesis was rejected at p<0.05.
In 2021, NEJM published the results of the DAPA-CKD study, which assessed the effect of dapagliflozin on the dynamics of renal function and the risk of death from cardiovascular disease or kidney pathology in patients with chronic kidney disease. The randomized placebo-controlled study included patients with an estimated glomerular filtration rate of 25-75 ml/min/1.73 m2 and a urine albumin/creatinine ratio of 200-5000 mg/g. The presence of diabetes mellitus was not a mandatory inclusion criterion. Patients received 10 mg dapagliflozin or placebo. A total of 4304 patients were included, with a median follow-up of 2.4 years. The study was stopped early due to the obvious advantages of dapagliflozin - patients receiving this drug had a significantly lower incidence of the primary endpoint “reduction in GFR by 50% or more/development of end-stage CKD/cardiovascular death/death from kidney disease”: 9.2% vs. 14.5%, OR 0.61; 95% CI 0.51 - 0.72; P<0.001; 19 patients must be treated to prevent one adverse event). Dapagliflozin was associated with a significant reduction in all-cause mortality (4.7% vs 6.8%, OR 0.69; 95% CI 0.53 to 0.88; P=0.004). Dapagliflozin also reduced the incidence of the combined endpoint “death from CVD/hospitalization due to decompensated CHF.” The effect of dapagliflozin was independent of the presence of concomitant diabetes mellitus. The safety profile of the drug was consistent with previously available data.
On April 30, 2021, the FDA issued a press release about the approval of Forxiga (dapagliflozin) for patients with chronic kidney disease. In addition to listing the benefits of using the drug in patients with CKD, the authors of the document remind of certain limitations of the use of Forxiga for these indications: the drug has never been tested (and is not expected to be effective) in patients with polycystic kidney disease (both autosomal dominant and recessive forms). inheritance), as well as in patients receiving or requiring immunosuppressive therapy for renal pathology. The document also lists clinically significant side effects of the drug: life-threatening cases of Fournier's gangrene, dehydration, urogenital infection, metabolic acidosis or ketoacidosis.
1) Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2021 Oct 8;383(15):1436-1446. doi: 10.1056/NEJMoa2024816. Epub 2020 Sep 24. PMID: 32970396.
2) Press release “FDA Approves Treatment for Chronic Kidney Disease” dated 04/30/21.
https://www.fda.gov/
Research results
The study included 27 patients (age 56 [49; 61] years), most of whom had T2DM lasting more than 4 years (7 [4; 12] years) and stage I obesity. Basic hypoglycemic therapy included a combination of metformin with sulfonylureas or dipeptidyl peptidase type 4 inhibitors (DPP-4 inhibitors).
The main characteristics of the patients and the dynamics of the studied markers of fibrosis and inflammation are presented in Table 1. Over 6 months. treatment with dapagliflozin there was a significant decrease in HbA1c levels from 8.2 [7.6; 8.8]% to 7.8 [7.2; 8.1]% (p=0.001), decrease in BMI and decrease in waist circumference. During the observation period, there were no significant changes in the content of lipoproteins and triglycerides, GFR and blood pressure.
The content of hsCRP and NT-proBNP did not differ from normal values and did not undergo statistically significant changes after 6 months. treatment with dapagliflozin. At baseline, patients had significantly higher concentrations of galectin-3, PICP and MMP-9 compared to the control group (p=0.017, p=0.006 and p=0.008, respectively). When assessing the dynamics of the content of galectin-3, MMP-9, TIMP-1, ST2, no significant differences with the initial values were obtained (see Table 1). At the same time, after 6 months. treatment with dapagliflozin there was a statistically significant decrease in PICP concentration from 136.8 [100.4; 200.6] ng/ml to 104.8 [79.7; 162.0] ng/ml (p=0.019) and an increase in TIMP-1 concentration from 188 [138; 270] ng/ml up to 234 [205; 315] ng/ml (p=0.011).
Discussion
The predominance of type I collagen synthesis over its degradation leads to the accumulation of excess collagen fibers in the myocardium and indicates the process of fibrosis that occurs in the interstitial and perivascular space, including in diabetic cardiomyopathy [9]. Levels of PICP, which is a marker of type I collagen formation and degradation, reliably correlate with the amount of collagen formed in both patients with and without chronic heart failure (CHF) [9].
Studies have shown that PICP concentrations are higher in patients with T2DM than in patients without T2DM [10]. Similar differences were demonstrated in our study. Therapy with dapagliflozin for 6 months. led to a significant decrease in P1CP levels compared to baseline. Similar dynamics were obtained in a study of another INGT2 in patients with T2DM and a very high risk of cardiovascular events [11].
Our study did not establish a significant decrease in NT-proBNP concentrations during treatment with dapagliflozin for 6 months, which can be explained by the population of patients included in the study without CHF and without high NT-proBNP concentrations. Since NT-proBNP is a marker of CHF and its level correlates with its severity, even in studies of patients with T2DM and CHF with preserved ejection fraction, no significant decrease in NT-proBNP concentration was detected [12]. However, the DAPA-HF trial demonstrated that the risk of worsening CHF or cardiovascular death was lower in the dapagliflozin group than in the placebo group [13]. Thus, despite having a limited effect on NT-proBNP levels, INGT2 improves clinical outcomes in patients with CHF, suggesting a discrepancy between short-term changes in NT-proBNP levels and clinical outcomes.
The concentrations of ST2, hsCRP, MMP-9 and its inhibitor TIMP-1 did not change significantly during dapagliflozin therapy, which is consistent with data from other studies [11, 14, 15]. Probably, the lack of significant dynamics in the indicators of a number of markers during our study is due to the fact that patients without CHF and cardiovascular events are at that stage of the cardiovascular continuum when the concentrations of markers cannot fully reflect the severity and prognosis of these patients. and their changes cannot be recorded over a 6-month observation period. In addition, the absence of negative dynamics in the content of these biomarkers may indicate a slowdown in the inflammatory and fibrotic processes that occur in T2DM and potentially translate into improved cardiovascular outcomes. It is also worth taking into account the presence of obesity and other unaccounted factors that may influence the values of the markers we evaluate.