The use of biphasic insulin aspart in the treatment of patients with type 2 diabetes mellitus
Diabetes mellitus (DM) type 2 is one of the most common chronic diseases and is a non-infectious epidemic. Currently, about 200 million people in the world suffer from this disease. The number of people with diabetes increases annually by 5–7% and doubles every 15 years. DM is characterized by early disability and high mortality of patients due to late vascular complications. This circumstance places this disease among the socially significant. Among patients with this pathology, patients with type 2 diabetes account for more than 90%.
The pathophysiological disorders in type 2 diabetes are based on two main defects: insulin resistance and pancreatic β-cell dysfunction (impaired insulin secretion and relative insulin deficiency). These two disorders are very closely related and first lead to increased blood glucose levels after meals (postprandial glycemia - PPG), and subsequently to fasting hyperglycemia. In addition, the sensitivity of peripheral tissues to insulin decreases.
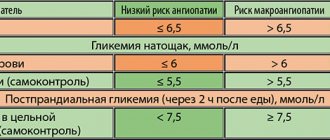
The International Diabetes Federation (IDF) has developed recommendations regulating therapeutic goals for type 2 diabetes (Table), based on which individual goals can be set to improve disease compensation.
| Table. Therapeutic goals for type 2 diabetes mellitus |
Strict glycemic control is of paramount importance to reduce the risk of micro- and macrovascular complications in type 2 diabetes. However, as the results of the UK Prospective Diabetes Study (UKPDS) show, as type 2 diabetes progresses, it becomes increasingly difficult to maintain optimal levels of fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) using only a hypocaloric diet and exercise. In this case, oral hypoglycemic drugs (OHDs) are prescribed. The main goal in the treatment of type 2 diabetes is to maintain blood sugar (glycemia) levels as close to normal as possible.
Currently, the following groups of PSSPs are used in the treatment of type 2 diabetes.
Drugs that enhance insulin secretion by the pancreas. This group of PSSPs includes sulfonylurea derivatives (PSM) - glibenclamide (Maninil), gliclazide (Diabeton MV), glimepiride (Amaril, Glemaz), gliquidone (Glyurenorm) and prandial glycemic regulators (glinides) - repaglinide (NovoNorm®). Each of the listed drugs has its own characteristics, mainly related to the duration of action. By increasing the stimulation of insulin by the pancreas, these drugs lower blood glucose levels.
Drugs that improve insulin sensitivity. This group of drugs includes biguanides and insulin sensitizers (thiazolidinediones). The main representative of the biguanide group is metformin (Glucophage, Siofor, Bagomet, Metfogamma). Metformin reduces the production of glucose by the liver by inhibiting gluconeogenesis and enhances the uptake of glucose by peripheral tissues without stimulating insulin secretion by the pancreas. Metformin is the drug of choice in the treatment of patients with type 2 diabetes and excess body weight. The drug is successfully used in combination with other PSSPs and insulin.
A subgroup of insulin sensitizers (thiazolidinediones) is represented by pioglitazone (Actos) and rosiglitazone (Avandia).
Drugs that reduce the absorption of carbohydrates in the intestines. This group includes acarbose (Glucobay), which is an inhibitor of α-glucosidases. Acarbose prevents the rise in blood glucose levels after meals by inhibiting the absorption of carbohydrates from food in the small intestine.
Combined drugs. They consist of several components, these include, for example, Glibomet (glibenclamide + metformin), Avandamet (rosiglitazone + metformin).
However, the effective action of many PSSPs occurs only when residual β-cell secretion is maintained. The progressive decline in β-cell function and insulin resistance characteristic of type 2 diabetes prevent the achievement of the desired effect even with combination therapy of several PSSPs. In this case, the best treatment option available is insulin therapy. One of the conclusions of the UKPDS study is: “We are not starting insulin therapy as early or as aggressively as needed” [2].
Maintaining the level of glycated hemoglobin within the recommended target values (< 7.0% - according to the recommendations of the American Diabetes Association; 6.5% - according to the recommendations of the International Diabetes Federation, the American Association of Clinical Endocrinology, the Ministry of Health and Social Development of the Russian Federation) reduces the risk of developing micro- and macrovascular complications of diabetes [5–8]. However, in recent years, there has been increasing evidence of the role of PPG as an independent risk factor for cardiovascular morbidity and mortality [9]. These data are very important in clinical practice, since patients with GKN levels close to target values may still have high levels of PPG [10, 11]. Indeed, it has been shown that when HbA1c < 7.3%, more than 70% of the total hyperglycemia is due to PPG [12]. Therefore, patients need to be prescribed more intensive treatment regimens aimed at controlling both GCH and PPG, educating them on the importance of targeted control of PPG and HbA1c levels [13]. Basal-bolus regimens with prandial administration of short-acting insulins and one or two injections of basal insulin are one of the possible options for intensifying insulin therapy to achieve recommended glycemic control goals. However, this option for starting insulin therapy is complicated due to the multiple injections required. In addition, basal-bolus therapy is not always indicated for patients in the early stages of type 2 diabetes with preserved residual β-cell function [14]. A single-dose regimen of basal insulin or a basal insulin analogue may also be an effective and practical option for starting insulin therapy when PSSP therapy alone no longer maintains satisfactory glycemic control [15, 16]. Although approximately 58% of patients on this regimen achieve a target HbA1c level of <7%, postprandial glycemic values may remain high [17, 18].
To improve the control of PPG while maintaining the BGN within the recommended values, insulin therapy using biphasic insulin analogues is optimal for patients with type 2 diabetes.
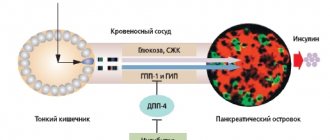
Biphasic insulin aspart (NovoMix® 30 - Novo Nordisk, Denmark) is a mixed dosage form containing 30% soluble and 70% protamine crystalline insulin aspart (Fig. 1). The first ultra-short-acting component reduces blood glucose levels after meals, and the second provides the basal (long-term) need for insulin. The combination of fast- and slow-acting components makes it possible to reproduce the profile of physiological insulin secretion. Therapy with NovoMix® 30 is indicated for patients with type 2 diabetes with unsatisfactory glycemic control while taking PSSP in sub- and maximum doses; with unsatisfactory control of PPG during therapy; in case of frequent, repeated hypoglycemia; patients who, for objective reasons, find it difficult to switch to intensive insulin therapy.
The potential benefits of using NovoMix®30 in patients with type 2 diabetes have been assessed in several international studies.
| Figure 1. Daily glycemic profiles in groups 1 and 2 of patients initially and after 3.5 months during therapy with NovoMix 30 |
The ACTION (Achieving Control Through Insulin plus Oral agents) study compared intensification of PSSP therapy with insulin therapy in a two-injection regimen [32]. The study lasted 24 weeks. It included patients with type 2 diabetes who failed to achieve target HbA1c values during PSSP therapy.
At the end of the study, 76% of patients were able to achieve an HbA1c < 7% in the NovoMix® 30 + PSSP group compared to 24% of patients receiving PSSP therapy alone. More patients with type 2 diabetes and unsatisfactory control during therapy with two PSSPs were able to achieve the recommended target HbA1c levels during therapy with NovoMix® 30 in combination with metformin and pioglitazone.
The study by Kabadi & Kabadi [27] examined the possibility of improving glycemic control in patients with type 2 diabetes when they were prescribed NovoMix® 30 insulin as a single injection in combination with metformin, PSM or both PSPS. It included 46 men with type 2 diabetes aged 45–70 years with an HbA1c level > 7.5%. The duration of the study was 6 months. At baseline, patients received either metformin alone (2500 mg/day), or glimepiride only (8 mg/day), or a combination of these drugs (2500 mg/day + 8 mg/day).
During the study, patients continued to receive their previous PSSP therapy with the addition of NovoMix® 30 (n = 38). 8 patients were transferred to placebo + NovoMix® 30 therapy with the abolition of PSSP. At baseline, body weight and HbA1c levels did not differ significantly between groups.
After 6 months, patients in all groups showed a significant decrease in HbA1c, including patients receiving NovoMix® 30 + placebo. Control goals (HbA1c < 7%) were achieved by patients in all groups. There were no episodes of severe hypoglycemia in any group.
Thus, single-injection therapy with NovoMix® 30 insulin provided all groups with the opportunity to achieve the target value for HbA1c < 7.0% and fasting plasma glucose (FPG) 5–7.2 mmol/l. A lower dose of insulin was observed during combination therapy with NovoMix® 30 with metformin and PSM.
The PREFER study [33] compared the effectiveness of two promising insulin analog therapy regimens: NovoMix® 30 in two injections and basal-bolus therapy (Levemir® + NovoRapid®). The duration of the study was 26 weeks. It included 715 patients with type 2 diabetes with a level of 7% < HbA1c < 12%, who had previously received PSSP therapy alone (72%) or in combination with insulin glargine (Lantus) or NPH insulin.
Insulin therapy NovoMix® 30 (n = 178) began with two injections per day (0.2 U/kg before breakfast and 0.1 U/kg before dinner). Basal-bolus therapy (n = 537) began with one injection of Levemir® insulin (10 units or 14 units if BMI > 32 kg/m2) plus prandial NovoRapid® insulin. Doses were selected individually in a ratio of 3: 2: 1 (for breakfast, lunch and dinner, respectively). PSSPs were abolished in both treatment groups.
Both insulin analog therapy regimens provided the opportunity to safely achieve the target HbA1c level of 7.0%: 50% in the NovoMix® 30 insulin therapy group and 60% in the Levemir® and NovoRapid® insulin therapy group in patients with type 2 diabetes. Patients who had not previously received insulin therapy achieved equal glycemic control during therapy with a biphasic analogue and basal-bolus therapy.
The average GKN value also turned out to be the same: 8.05 mmol/l during therapy with NovoMix® 30 insulin and 8.27 mmol/l during basal-bolus therapy with insulin Levemir® and NovoRapid® (p = 0.345). The incidence of hypoglycemia was low and comparable for both insulin analog therapy regimens.
The aim of the randomized, open-label, parallel-group EuroMix study [24] was to compare the safety efficacy of two insulin analogue therapy regimens in 255 patients with type 2 diabetes: insulin NovoMix® 30 in a two-injection regimen in combination with metformin (n = 128) and insulin Lantus as a single injection in combination with glimepiride once a day (n = 127). The primary study parameter was the difference in the dynamics of HbA1c reduction between groups after 26 weeks of therapy.
| Figure 2. Dynamics of prandial increase in glycemia initially and after 3.5 months during therapy with NovoMix 30 |
The study showed that starting insulin therapy with the use of the biphasic analogue NovoMix® 30 in a two-time regimen in combination with metformin provides a more pronounced decrease in HbA1c levels and the average prandial increase in glycemia in patients with type 2 diabetes compared to therapy with the insulin analogue Lantus® in a single injection regimen in combination with glimepiride.
We conducted a study in 10 administrative districts of Moscow to study the effectiveness of the drug NovoMix® 30 in everyday clinical practice for 3.5 months in 3,000 patients with type 2 diabetes with unsatisfactory glycemic levels during previous therapy.
For the final analysis, 309 questionnaires were selected by random sampling (every 10th questionnaire). The duration of type 2 diabetes was 11.5 ± 1.5 years. Before transferring to NovoMix® 30, patients received monotherapy with PSSP or combination therapy with PSSP and insulin. The HbA1c level in the total group of patients was initially 9.3 ± 1.5%. During the study, patients were divided into two groups: in the 1st group, patients received NovoMix® 30 in two injections in combination with PSSP, in the 2nd group - NovoMix® 30 in three injections without PSSP.
| Figure 3. Dynamics of prandial increase in glycemia initially and after 3.5 months during therapy with NovoMix® 30 |
Group 1 consisted of 250 patients (195 women/55 men), the average duration of the disease was 11.5 ± 6.2 years, the initial HbA1c level was 9.3 ± 1.8%, FCL 9.9 ± 2.3 mmol/ l, PPG 11.7 ± 2.7 mmol/l. This group received NovoMix® 30 in two injections before breakfast and before dinner in combination with PSSP, represented by sulfonylurea drugs and metformin in maximum doses.
Group 2 included 59 patients (44 women/15 men), with an average disease duration of 11.7 ± 7.8 years, initial HbA1c levels of 9.4 ± 1.8%, FCL 9.5 ± 2.5 mmol/l, PPG 11.3 ± 2.6 mmol/l. This group of patients received NovoMix® 30 in a regimen of three injections before main meals.
The analysis data showed that during observation in both groups, the daily glycemic profile (8 points), determined during self-monitoring (p < 0.02) (Fig. 1, 2), and HbA1c significantly improved.
There was a significant decrease in the average prandial increase in glycemia (average increase in glycemia from preprandial at 90 minutes after a meal; p < 0.04) in comparison with the initial values (p < 0.01) in both groups of patients (Fig. 3).
| Figure 4. Dynamics of HbA1c at baseline and after 3.5 months during therapy with NovoMix® 30 |
By the end of the study, in both groups of patients, the HbA1c level decreased to 7.5 ± 0.8% (Fig. 4).
Thus, data on the use of the biphasic insulin analogue NovoMix® 30 in the treatment of patients with type 2 diabetes allow us to draw the following conclusions.
- Starting therapy with one injection of NovoMix® 30 in addition to PSSP makes it possible to effectively achieve target disease control.
- Intensification of NovoMix® 30 therapy to two or three injections allows achieving the target level in most patients without increasing the risk of hypoglycemia.
- Control of PPG and improvement of the lipid profile during therapy with the biphasic analogue NovoMix® 30 may potentially reduce the risk of cardiovascular complications.
- Control of PPG is an important component of diabetes management.
- Insulin therapy must not only be started in a timely manner, but also intensified in a timely manner.
Literature
- Riddle MC Tactics for Type 2 diabetes. Endocrinol Metab Clin North Am. 1997; 26:659–677.
- Turner RC, Cull CA, Frighi V. et al. For the UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281: 2005–2012.
- Chan JL, Abrahamson MJ Pharmacological management of Type 2 diabetes mellitus // Mayo Clin Proc. 2003; 78:459–467.
- Weyer C., Bogardus C. et al. // J. Clin Invest. 1999; 104:787–794.
- Stratton IM, Adler AJ, Neil HA et al. UKPDS 35: Prospective observational study // Br Med J. 2000; 321:405-412.
- European Diabetes Policy Group 1999. A desktop guide to type 2 diabetes mellitus. Diabet Med. 1999; 16: 716-730.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005; 28 (Suppl1): 4-36.
- American College of Clinical Endocrinologists. Medical guidelines for the management of diabetes mellitus. 2002 update. Endocr Pract. 2002; 8(Suppl 1): 40-83.
- Gerich JE Arch Intern Med. 2003; 163: 1306-1316.
- Ceriello A. Diabetologia. 2003; 46(Suppl 1): 9–16.
- Monnier L., Lapinski H., Collette C. Diabetes Care. 2003; 26:881–885.
- Landgraf R. Diabet Vetab Res Rev. 2004; 20(Suppl 2): 9–12.
- Abrahamson MJ Arch Intern Med. 2004; 164:486–491.
- Lebovitz HE Diabetes Rev. 1999; 7: 139-153.
- Riddle MC Am J Med. 2004; 116(Suppl 3A): 10–16.
- Rosenstock J Am J Med. 2004; 116(Suppl 3A): 10–16.
- Riddle MC, Rosenstock J, Gerish J. Diabetes Care. 2003; 26:3080–3086.
- Fritsche A., Schweitzer MA, Haring HU Ann Intern Med. 2003; 138:952–959.
- Bell DS, Clements RS et al. Arch Intern Med. 1991; 151: 2265-2269.
- Owens DR, Zinman B, Bolli GB Lancet. 2001; 358: 739-746.
- Heller S. Int J Obes. 2002; 26(Suppl 3): 31-36.
- Bolli GB, Di Marchi RD, Park GD et al. Diabetologia. 1999; 42: 1151-1167.
- Weyer C., Heise T., Heinemann L. Diabetes Care. 1997; 20: 1612-1614.
- Lund et al. Diabetologia 2006; 49(Suppl 1): 600.
- Garber et al. Diabetes Obes Metab. 2006; 8: 58-66.
- Kilo et al. J Diabetes Complications. 2003; 17: 307-13.
- Kabadi & Kabadi. Diabetes Res Clin Prac. 2006; 72(3): 265-270.
- Naiker et al. Diabetologia. 2006; 49(Suppl 1): 599.
- Gasher B. Hirsch I. Arch Intern Med. 1998; 158: 134-140.
- Ohkubo Y. Kishikawa H. et al. Diabetes Res Clin Pract. 1995; 28: 103-117.
- Hermansen K., Vaaler S., Madsbad S. Metabolism. 2002; 51 (7): 896-900.
- Raskin et al. Diabetes. 2006; 55 (Suppl 1): 131.
- Liebl et al. Diabetes. 2006; 55 (Suppl 1): 123.
M. B. Antsiferov , Doctor of Medical Sciences, Professor L. G. Dorofeeva Endocrinological Dispensary of the Moscow Department of Health
Detailed description of the study
Insulin is a hormone produced by B cells of the pancreas, which reduces the concentration of glucose in the cell and stimulates the synthesis of proteins and fats. Determination of insulin concentration is used to determine the type of diabetes mellitus, select a therapeutic drug, and determine the degree of pancreatic B-cell insufficiency.
An increase in insulin levels is observed in the following diseases: obesity, type 2 diabetes mellitus, normal pregnancy, acromegaly, Itsenko-Cushing syndrome, insulinoma. With a decrease in insulin concentration, type 1 diabetes mellitus develops, as well as type 2 diabetes mellitus with insulin requirement.
Insulin regulates carbohydrate metabolism, maintaining blood glucose at the required level, and also participates in fat (lipid) metabolism.
The concentration of insulin in the blood directly depends on the concentration of glucose: after eating, a large amount of glucose enters the blood, in response to this the pancreas secretes insulin, which triggers mechanisms for moving glucose from the blood into the cells of tissues and organs. Insulin also regulates biochemical processes in the liver: if there is a lot of glucose, the liver stores it in the form of glycogen (a glucose polymer) or uses it for the synthesis of fatty acids. When insulin synthesis is impaired and its concentration is reduced, glucose cannot enter the body's cells and hypoglycemia develops. Cells lack glucose, the main substrate for energy production. If this condition is chronic, then metabolism is disrupted and pathologies of the kidneys, cardiovascular, and nervous systems begin to develop, and vision suffers.
Diabetes mellitus is a disease in which there is a lack of insulin production. Diabetes is of types 1 and 2. The first type develops when the pancreas produces insufficient insulin, the second type (the most common) - when cells lose sensitivity to the effects of insulin on them.
In the initial stages, diabetes mellitus is treated with a special diet and medications that enhance the production of insulin by the pancreas or stimulate the body's cells to consume glucose by increasing their sensitivity to this hormone. When the pancreas stops producing insulin, it requires injection.
An increased concentration of insulin in the blood is called hyperinsulinemia. At the same time, the glucose content in the blood decreases sharply, which can lead to hypoglycemic coma and even death, since the functioning of the brain depends on the concentration of glucose. Elevated levels of insulin in the blood can also be caused by the presence of a tumor that secretes it in large quantities - insulinoma. In this case, the concentration of insulin in the blood can increase tens of times within a short time. Diseases associated with the development of diabetes mellitus: metabolic syndrome, pathology of the adrenal glands and pituitary gland, polycystic ovary syndrome.