- Diabetes mellitus (DM) is a public health problem affecting approximately 382 million people worldwide.
- In order to achieve target glycemic levels, insulin therapy is the first line of therapy for patients with type 1 diabetes; For patients with type 2 diabetes, the use of insulin therapy is an option for both initial and additional therapy.
- Despite the development of insulin therapy in recent decades, several obstacles remain to the administration and optimal use of insulin in clinical practice: fear of hypoglycemia,
- weight gain,
- pain associated with blood tests
- pain from injections.
Development of insulin therapy
- About 382 million people worldwide suffer from diabetes mellitus (DM), type 2 DM accounts for 85-95% of all cases.
- For patients with type 2 diabetes, the use of insulin therapy is an option as both initial and additional therapy for those who have not achieved glycemic control when taking oral glucose-lowering drugs.
Insulin was first used in diabetic patients in the 1920s, but the first commercial preparations contained various impurities and associated potential complications.
In 1930, protamine-zinc insulin was developed, which resulted in delayed absorption and a longer duration of action, thereby reducing the number of insulin doses required for insulin replacement therapy.
Neutral protamine Hagedorn (NPH), the main basal insulin throughout the 20th century, was produced in 1946.
In the early 1980s, new long-acting insulin analogues, Glargine and Detemir, appeared, which were characterized by:
- less variability of glycemia during the day,
- longer duration of action,
- administration - 1 time per day.
The search for the ideal insulin regimen is still ongoing, striving to provide optimal glycemic control with minimal side effects and improvement in patient condition.
Overdose of the drug Insulin glargine, symptoms and treatment
Symptoms: initial symptoms of hypoglycemia are a sudden increase in sweating, palpitations, tremors, hunger, agitation, paresthesia in the mouth, pallor, headache, sleep disturbances. In severe cases of overdose - coma. Treatment: The patient can eliminate mild hypoglycemia himself by taking sugar or sugar-rich foods. In severe cases, 1 mg of glucagon is administered subcutaneously or intramuscularly. If necessary, therapy is continued with intravenous administration of concentrated glucose solutions. Long-term medical observation is required, since after visible clinical improvement, recurrence of hypoglycemia is possible.
List of pharmacies where you can buy Insulin glargine:
- Moscow
- Saint Petersburg
Pharmacokinetic and pharmacodynamic profiles of insulin therapy
- The development of new insulins is aimed at reducing episodes of hypoglycemia in patients and improving the pharmacokinetic (PK)/pharmacodynamic (PD) profile.
- Inconsistency in the PK profile among insulins directly affects PD effects, increasing the likelihood of hypoglycemic episodes due to difficult to predict insulin peaks.
To minimize episodes of hypoglycemia or hyperglycemia, insulin analogues with different PK/PD profiles have been developed, including basal and ultra-long basal insulins.
Requirements for an ideal basal insulin:
- stable PD profile,
- low risk of hypoglycemia,
- duration of action 24 hours,
- low individual variability of action.
Comparison between the action time profiles of different insulins.
- Insulin-NPH is characterized by: slow absorption,
- peak 4.5 hours after administration,
- duration of action is less than 24 hours, so it is usually administered at least twice a day,
- high individual variability.
- After subcutaneous administration, glargine remains in the subcutaneous tissue.
- PK/PD profile similar to Glargine: smooth PK/PD profile,
Both Glargine and Detemir cannot completely mimic physiological basal insulin secretion.
- When taken orally in large doses, both exhibit a peak PK/PD profile;
- low doses may not be sufficient to cover a 24-hour period;
- There is also individual variability of action.
To try to overcome this problem, ultra-long-acting insulins such as Degludec, LY2605541 and Glargine 300U have been developed.
Degludec
- Insulin Degludec has a modified B-chain. Like other insulins, Degludec forms hexamers and dihexamers when administered subcutaneously.
- Slow diffusion of the zinc molecules present in the hexamer occurs and the insulin monomer is absorbed.
- Compared to insulins Glargine and Detemir, Degludec has a smooth PK/PD profile, which reduces the number of episodes of hypoglycemia.
- Insulin Degludec has a half-life of more than 25 hours (action time >42 hours), the most studied route of administration in clinical studies is 1 dose once a day.
Insulin LY2605541
- Insulin LY2605541 (or PEGylated Lispro) has a polyethylene glycol (PEG) polymer chain in its molecule structure.
- PEGylation slows the subcutaneous absorption of LY2605541 and reduces its renal excretion, promoting a peak-free PK/PD profile,
- increase in half-life of LY2605541.
- weight loss,
Glargine 300 units
- The mechanism of absorption is the same as that of insulin glargine.
- When administered subcutaneously, Glargine 300U is deposited over a smaller area, creating a prolonged release, which leads to a more even PK/PD profile than glargine 100U.
- The effect of Glargine 300 units is more uniform and glucose control is maintained for 36 hours, which leads to a decrease in the frequency of hypoglycemic episodes (in general, and at night in particular) in patients taking only insulin or receiving insulin in combination with oral hypoglycemic drugs.
- Glargine 300U also showed reduced variability and a trend toward weight loss compared to Glargine 100U.
Insulin glargine concentration 300 units versus 100 units over time.
Insulin therapy should mimic physiological insulin secretion,
- thus, a longer half-life of insulin is not always the best choice.
As an example:
- Insulin Ultralente "VO-S" has an average duration of action of 20 hours, which exceeds the 14 hours of NPH insulin.
- But this insulin is characterized by: higher variability,
- high frequency of hypoglycemia episodes,
- worse glucose control compared with NPH.
Ultra-long insulins are characterized by:
- high efficiency,
- security,
- less pharmacodynamic variability,
- lasting more than 24 hours.
Among these insulins, both Glargine 300 and Degludec can be used as basal and basal-bolus therapy for types 1 and 2 diabetes.
Drug interactions Insulin glargine
The hypoglycemic effect of insulin is enhanced by MAO inhibitors, oral hypoglycemic drugs, ACE inhibitors, fibrates, disopyramide, fluoxetine, pentoxifylline, propoxyphene, salicylates and sulfonamides. The hypoglycemic effect of insulin is reduced by danazol, diazoxide, diuretics, glucagon, corticosteroids, isoniazid, estrogens, gestagens, fe derivatives nothiazine , somatotropin, sympathomimetics and thyroid hormones. Clonidine, β-blockers, lithium salts and ethanol can both enhance and weaken the hypoglycemic effect of insulin. Pentamidine can cause hypoglycemia, which in some cases is replaced by hyperglycemia. Under the influence of drugs with sympatholytic action, such as β-blockers, clonidine, guanfacine and reserpine, signs adrenergic counterregulation may be reduced or absent.
Clinical studies evaluating ultra-long-acting insulin formulations
Insulin Degludec for type 1 diabetes
- BEGIN was an open-label, single-arm, multicenter study evaluating adult patients with type 1 diabetes who were treated with basal-bolus insulin for at least 1 year (70% receiving insulin Glargine and 19% receiving insulin Detemir).
- A total of 626 participants were randomized
- in a 3:1 ratio of daily intake of insulin Degludec (100 units/ml; 3 ml) or insulin Glargine 100 units/ml; 3 ml),
- in combination with the use of insulin Aspart for food.
- Insulin Degludec demonstrated superiority in reducing glycated hemoglobin levels by 0.4% compared to 0.39% for Glargine (difference -0.01%; 95% CI -0.14–0.11; p < 0.0001).
- a significant reduction in the incidence of nocturnal hypoglycemia by 25% (95% CI 5 to 41%),
A follow-up study (BEGIN: Flex T1) tested the efficacy and safety of once-daily administration of insulin Degludec depending on the timing of its administration.
- This was an open-label clinical trial comparing timed administration of insulin Degludec (100 U/mL) (with a minimum of 8 hours and a maximum of 40 hours between doses).
- administration of insulin Degludec (100 units/ml; 3 ml) or Glargine (100 units/ml; 3 ml) at the same time once every day.
Insulin Degludec for type 2 diabetes
The BEGIN study of basal-bolus insulin treatment for type 2 diabetes was a randomized, phase 3a, open-label trial conducted at 123 sites in 12 countries.
- The study included adult patients with type 2 diabetes who underwent any course of insulin therapy for at least 3 months with or without oral hypoglycemic drugs and a glycated hemoglobin concentration of 7.0–10.0%.
- The study included 1006 patients randomized 3:1 to once-daily administration of insulin Degludec (100 U/mL; 3 mL) or Glargine (100 U/mL; 3 mL) in combination with insulin Aspart, with or without food. without prescribed metformin, pioglitazone, or both.
- The main objective was to confirm the superiority of Degludec over Glargine in terms of reducing glycated hemoglobin levels.
- After 1 year, the level of glycated hemoglobin decreased by 1.1% in the Degludec group and by 1.2% in the Glargine group (degludec-glargine difference: 0.08%; 95% CI -0.05 - 0.21), which proved its lack of benefit in reducing glycated hemoglobin.
- The incidence of hypoglycemia was lower in those receiving Degludec (11.2 vs. 13.6 episodes/patient-year; estimated ratio 0.82; 95% CI 0.69–0.99), including nocturnal hypoglycemia (1.4 vs. 1.8 episodes/patient-year; estimated ratio 0.75 ; 95% CI 0.58–0.99).
- Cases of severe hypoglycemia were extremely rare in both groups.
- The incidence of side effects was similar in both groups.
- A 26-week extension study showed that Degludec led to similar improvements in glycemic control compared to Glargine, with fewer hypoglycemic episodes (24% fewer overall and 31% fewer nighttime episodes).
Another large one-year phase 3 study (BEGIN Once Long) randomized 1030 patients with type 2 diabetes
- have not previously received insulin,
- with insufficient compensation with oral hypoglycemic agents (level of glycated hemoglobin 7-10%),
- some of whom received insulin Degludec (3:1), and others Glargine once a day,
- both insulins in combination with metformin.
- Insulin Degludec showed similar indicators of glycemic control,
- reduced risk of developing nocturnal hypoglycemia,
- reduced risk of severe side effects.
Flexible insulin dosing schedule Degludec has also been studied in patients with type 2 diabetes. BEGIN Flex Study:
- included 687 patients (41.8% already receiving insulin),
- randomized to administer Degludec once a day at different times (with a set dose of administration within a time interval of 8-40 hours),
- Degludec once a day before the evening main meal,
- Glargina.
- glycemic control,
Insulin glargine 300 units/ml for type 1 diabetes
The EDITION 4 phase 3A study, the results of which were published in 2015, was randomized and open-label.
- The study randomized 549 patients (1:1:1:1) to receive once daily insulin glargine 300 units in the morning,
- insulin glargine 300 units in the evening,
- insulin glargine 100 units in the morning,
- insulin glargine 100 units in the evening.
- HbA1C levels changed from baseline, decreasing by 0.40 and 0.44%, respectively (0.04; 95% CI -0.10 to 0.19%).
Insulin glargine 300 units/ml for type 2 diabetes
- The EDITION 1 study was a 6-month, multicenter (conducted in 13 countries), open-label, which included: adult patients with type 2 diabetes,
- receiving basal-bolus insulin therapy (≥42 units/day basal insulin) for at least one year (57.4% using metformin; use of other oral glucose-lowering drugs was not permitted)
- level of glycated hemoglobin 7.0–10.0%.
- insulin glargine 300 units once a day,
- Patients receiving Glargine 300 units achieved better results in reducing the risk of hypoglycemia by 21% (relative risk 0.79; 95% CI 0.67–0.93).
The phase 3 study EDITION 2 was randomized, with a design similar to EDITION 1:
- this was a 6-month, open-label study,
- carried out in 213 centers in 13 countries,
- analyzing adult patients with type 2 diabetes who received basal insulin therapy (≥42 units/day basal insulin) for at least 1 year,
- had a glycated hemoglobin level of 7.0–10.0%.
- did not receive short-acting insulin before meals,
- insulin injections Glargine 300 units once a day,
- Results were statistically comparable in both groups (mean change -0.57% for those who administered 300 units of glargine and -0.56% for those who administered 100 units of glargine).
- Lower in patients receiving Glargine 300 units (21.6%) than Glargine 100 units (27.9%), with a risk reduction of 23% (relative risk 0.77; 95% CI 0.61–0.99).
- Incidence/participant-year evidence of chronic or severe nocturnal hypoglycaemia was 37% lower with insulin glargine 300 units (1.74 vs 2.77; RR = 0.63; 95% CI 0.42–0.96).
EDITION 3 is another multicenter randomized trial evaluating insulin Glargine 300 units, but evaluating insulin therapy in adult patients with type 2 diabetes on oral glucose-lowering drugs.
- Participants (N = 878) were randomized to receive insulin glargine 300 units once daily.
- insulin Glargine 100 units once a day for 6 months after stopping taking sulfonylureas and glinides.
- The reduction in HbA1C was similar in both groups (mean change -1.42% in those who administered 300 units of glargine and -1.46% in those who administered 100 units of glargine; mean difference 0.04%; 95% CI -0.09% to 0.17% ).
- Results were statistically similar in patients receiving Glargine 300 units (16%) or Glargine 100 units (17%).
Flexible interval insulin administration Glargine 300 units has also been studied in patients with type 2 diabetes.
- Patients participating in EDITION 1 (N = 109) and EDITION 2 (N = 89) using glargine 300 units were randomized at month 6: one group continued the fixed dosing regimen,
- the other switched to a flexible injection schedule, allowing 24 ± 3 hours between injections on at least 2 days a week.
Barriers to insulin therapy and unmet needs: focus on hypoglycemia
Several barriers remain to the adoption of insulin and its optimal use in clinical practice, both at the patient and clinician level. The most common reasons for refusing to start insulin therapy in patients with type 2 diabetes are:
- fear of hypoglycemia,
- weight gain,
- discomfort associated with the need for regular blood tests,
- pain from injections,
- the opinion that insulin therapy is a complex and labor-intensive process.
Adherence to insulin therapy, however, is more influenced by factors such as health insurance structure and employment.
Fear of hypoglycemia not only influences the decision to initiate insulin therapy, but it may also compromise the adequacy of glycemic control.
It has been reported that most physicians would treat patients in a more aggressive manner without adequate glucose control if not for concerns about hypoglycemia.
Burden of hypoglycemia in patients with diabetes receiving insulin therapy
Hypoglycemia is a significant side effect of diabetes therapy, as it
- worsens the quality of life of patients.
Observational studies show that
- hypoglycemia occurs in 42.89 cases/patient-year with type 1 diabetes,
- 16.36 cases/patient-year in patients with type 2 diabetes receiving insulin therapy.
The incidence of severe hypoglycemia is approximately
- 1.15 cases/patient-year and can reach 3.2 cases/patient-year for type 1 diabetes;
- 0.7 cases/patient-year in patients with type 2 diabetes receiving insulin therapy.
Nocturnal hypoglycemia that occurs during sleep is especially dangerous because patients are unlikely to recognize the symptoms or wake up during the episode.
- The DCCT study reported that 43% and 55% of all hypoglycemic and severe hypoglycemic events, respectively, occurred during sleep.
- A large online survey conducted in the United States among 7,239 participants with type 2 diabetes (28.7% on insulin therapy) showed that hypoglycemia interfered with social activity,
- caused more absenteeism,
- decrease in overall labor productivity,
- negatively affected the overall quality of life.
- with losses ranging from U$ 15.26 to U$ 93.47
- 2108 respondents (32.8% with type 1 diabetes and 67.2% with type 2; 74.2% receiving insulin) reported that the frequency of hypoglycemic episodes significantly affected sleep and functional status the next day, 60.7% reported moderate to severe consequences .
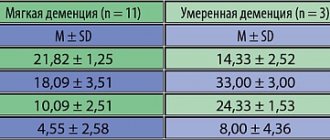
- A longitudinal study of 16,667 older patients with type 2 diabetes (mean age 65 years; 35% receiving insulin) after 27 years showed an increased risk of dementia among patients with hypoglycemic episodes, with risk increasing as the number of hypoglycemic episodes increased.
The frequency of hypoglycemic episodes may lead patients to avoid taking medications, with significant reductions or even discontinuation of insulin doses after a hypoglycemic event, which negatively affects glycemic control.
Side effects of the drug Insulin glargine
Associated with the effect on carbohydrate metabolism: hypoglycemic conditions (tachycardia, increased sweating, pallor, hunger, irritability, convulsions, confusion or loss of consciousness). Local reactions: lipodystrophy (1–2%), skin hyperemia, itching, swelling at the injection site. Allergic reactions: urticaria, Quincke's edema, bronchospasm, arterial hypotension, shock. Other: transient refractive errors, progression of diabetic retinopathy (with sharp fluctuations in blood glucose levels), edema. Most minor reactions at the insulin injection site resolve within a few days (several weeks) from the start of therapy.


![Table 3. Pharmacokinetic and pharmacodynamic parameters of fluoroquinolones with a single standard dose taken orally [7, 13]](https://irknotary.ru/wp-content/uploads/tablica-3-farmakokineticheskie-i-farmakodinamicheskie-parametry-ftorhinolonov-pri-odnokratnom-prieme-330x140.jpg)