Detailed description of the study
Tacrolimus is an immunosuppressant that prevents organ rejection during organ transplantation. After transplantation, a person’s body is weakened, so it is necessary to carefully select the dose of immunosuppressants so that, on the one hand, they act, and on the other, do not cause side effects. Tacrolimus refers to drugs, even a slight change in the concentration in the blood of which can lead to treatment failure, toxic tissue damage, or excessive suppression of the immune system and the addition of infections. In order to avoid such phenomena, an analysis is carried out to determine the content of tacrolimus in plasma.
When an organ is transplanted, it is considered foreign by the body, and immune cells begin to attack it, which can lead to rejection. Tacrolimus inhibits the action of the enzyme calcineurin, thereby reducing the formation and activity of immune cells. This protects the transplant and the body as a whole from destruction by its own immunity.
When taking the drug, side effects from different systems may occur. Thus, ischemia, increased blood pressure, arrhythmias, and bleeding are observed in the heart and blood vessels. Anemia, headaches, muscle twitching, hearing and vision impairment, and metabolic disorders are also noted. Taking the drug increases the risk of viral and bacterial infections and may be a factor provoking the formation of benign and malignant tumors. The medicine also affects the general condition: a person may feel weak, the temperature rises, and swelling appears.
In case of an overdose of the drug, encephalopathy may occur. It manifests itself as headaches, convulsions, and mental disorders. If the use of tacrolimus is stopped in a timely manner, in most cases the condition resolves without consequences.
The drug is used for liver and kidney transplants. Tacrolimus takes a long time to reach therapeutic concentrations in the blood, so its content is assessed on average three days after dose adjustment. The drug is processed primarily in the liver and intestinal wall, and then excreted from the body in urine and feces.
The dose of the drug is selected individually and may be different at certain periods both in different patients and in one person. Proper blood concentration not only protects the organ from rejection, but also minimizes serious health consequences due to overdose. Therefore, regular monitoring of the concentration of tacrolimus in the blood should be carried out, especially when adjusting the dose, changing the treatment regimen, prescribing new drugs, and transplant rejection reactions.
Tacrolimus - pharmacokinetics and modern aspects of clinical use
Immunological rejection reactions during organ and tissue transplantation are one of the most serious problems affecting the quality of treatment, life, morbidity and survival of patients. Although these reactions differ in direction during solid organ transplantation and allogeneic hematopoietic stem cell transplantation (AHSCT), their prevention and treatment is facilitated by the use of similar immunosuppressive therapy regimens in the posttransplantation period.
When transplanting solid organs (kidneys, liver, heart, lungs, etc.), an organ rejection reaction develops, caused by the recipient’s immune system. As a rule, in this case, the rejection reaction is rather chronic in nature, and tolerance to the transplanted organ occurs relatively quickly, which allows for moderate immunosuppression. Whereas during AHSC transplantation, where two systems are transplanted from the donor - immune and hematopoietic, reactions are directed to all other organs and systems of the recipient and their severity depends on the degree of incompatibility with HLA antigens. In case of obvious incompatibility, acute and chronic graft-versus-host reaction occurs in 100% of cases, is pronounced and requires maximum and long-term immunosuppression.
The synthesis and clinical introduction of calcineurin inhibitors made it possible to perform any type of transplantation and significantly reduced both the number of acute and chronic immunological reactions and the number of side effects, the frequency of which was extremely high in early regimens using steroids and azathioprine [1].
Tacrolimus is one of the relatively new immunosuppressive drugs, discovered in 1984 by Japanese researchers, which has shown clinical efficacy comparable to the previously used cyclosporine A in most types of transplantations, but with less renal and neurological toxicity. The absence of hepatoenteric recirculation and, accordingly, more predictable kinetics have made tacrolimus the drug of choice for liver transplantation. This article is devoted to some aspects of pharmacokinetics, describing the mechanism of action, side effects and analyzing the use of tacrolimus in clinical practice.
Mechanism of action
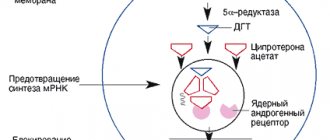
Tacrolimus, a cyclic macrolide, was isolated from microorganisms (Streptomyces tsukubaensis) [2]. Despite this, its antimicrobial activity is negligible. This drug is capable of binding at the molecular level to the cytosolic protein FKBP12, which is responsible for the intracellular accumulation of the drug. The FKBP12–tacrolimus complex specifically and competitively binds to and inhibits calcineurin, which in turn blocks phosphorylation of the cytoplasmic component required for transcription of the interleukin-2 (IL-2) gene. The mechanism of action of tacrolimus also suggests that it affects not only the production of IL-2, but also suppresses other “early” T-cell activation genes that control the production of cytokines such as IL-3, granulocyte-monocyte colony-stimulating factor , TNF-alpha, interferon gamma, etc., which leads to calcium-dependent inhibition of T-cell signaling transduction pathways, thus preventing the transcription of a discrete group of lymphokine genes [3, 4]. The ability of tacrolimus to inhibit the activity of T lymphocytes in vitro is 100 times greater than that of cyclosporine A (inhibitory concentration IC50 - 0.1 nmol/l and 10 nmol/l, respectively).
Pharmacokinetics
Tacrolimus is absorbed from the gastrointestinal tract, the main site of absorption being the upper gastrointestinal tract. Concentrations (Cmax) of tacrolimus in the blood reach a peak after approximately 1-3 hours, bioavailability is 14-17% in healthy volunteers and varies greatly in patients after solid organ transplantation and HSCT - from 4 to 89% (average 25%). In some patients, the drug is continuously absorbed over a long period, achieving a relatively flat absorption profile. Following oral administration of the drug (0.30 mg/kg/day) to liver transplant patients, most patients achieved steady-state tacrolimus concentrations within 3 days. When used simultaneously with food, the rate and degree of absorption of tacrolimus decreased. Bile secretion does not affect the absorption of tacrolimus, so there is no need to recalculate the dose of tacrolimus in patients with bile flow obstruction. There is a strong correlation between the area under the pharmacokinetic curve and whole blood drug trough levels at steady state, and monitoring blood trough levels may serve to adequately assess systemic drug exposure.
Distribution
The distribution of tacrolimus after intravenous administration of the drug to humans is biphasic. In blood plasma, the drug is highly bound (75–99%) to proteins, mainly serum albumin, α-1-acid glycoprotein, lipoprotein and globulins [5]. In the systemic circulation, tacrolimus is largely bound to red blood cells due to their high content of FKBP. Significant concentrations of tacrolimus are found in the lungs, spleen, heart, kidneys, liver, pancreas and brain. The volume of distribution ranges from 0.85 to 65 l/kg [6]. Tacrolimus is a drug with low clearance. In adult patients with liver and kidney transplants, the values of this parameter were 4.1 l/h and 6.7 l/h, respectively. In children with a liver transplant, the total clearance value is approximately 2 times higher than in adult patients. The half-life of tacrolimus is long and variable. In adult and pediatric liver transplant patients, the half-life averaged 11.7 hours and 12.4 hours, respectively, compared with 15.6 hours in adult kidney transplant patients.
Metabolism and excretion
Tacrolimus is metabolized through the cytochrome P450IIIa4 system in the liver and possibly in the small intestine. Only 1 metabolite out of 8 has immunosuppressive activity. The drug is excreted by the liver. Less than 1% of the administered dose is found in the urine [6].
Indications, methods of use and dosage
Tacrolimus is used to prevent and treat rejection in solid organ transplantation, acute and chronic graft-versus-host disease, as well as for the treatment of atopic dermatitis, eczema and psoriasis (in cream form). Tacrolimus can be used either orally or intravenously.
Oral tacrolimus therapy in adults should be initiated at a dosage of 0.1–0.2 mg/kg/day, divided into 2 divided doses. The use of the drug should begin approximately 12 hours after completion of the operation. If the patient’s condition does not allow taking the drug orally, intravenous therapy should be started with a dosage of 0.01–0.05 mg/kg/day, administering the drug as an intravenous extended infusion over 24 hours or 2 times a day for 4 hours. In children the initial dose is 0.03–0.06 mg/kg/day.
During maintenance therapy, the dosage of tacrolimus should be adjusted depending on the individual needs of the patient, taking into account the results of monitoring the levels of the drug in the patient's blood. The therapeutic concentration of the drug in the blood is considered to be 5–15 ng/ml. At concentrations greater than 20 ng/ml, the risk of developing nephrotoxicity increases significantly.
Side effects
Tacrolimus causes side effects common to all calcineurin inhibitor drugs. Neurotoxicity, nephrotoxicity, impaired glucose metabolism and hypertension occur most frequently (>1/100) [7, 8]. Electrolyte disturbances (hypomagnesemia, hypokalemia), thrombotic thrombocytopenic purpura and lymphoproliferative diseases are observed much less frequently. Unlike cyclosporine A, tacrolimus rarely causes significant liver toxicity, hirsutism, or gingival hyperplasia. The severity of side effects significantly depends on the premorbid background, the combined use of other immunosuppressants, the presence of somatic pathology and the toxicity of the treatment. Thus, toxicity data for patients after AHSCT transplantation differ significantly from data after solid organ transplantation.
The nephrotoxicity of calcineurin inhibitors is due to the fact that drugs in this group reduce the concentration of renin and aldosterone, increase the resistance of renal vessels, reducing glomerular filtration and renal blood flow [9]. Comparative randomized studies have shown that tacrolimus has significantly less nephrotoxicity than cyclosporine A. In addition, hypertension and hyperlipidemia were observed significantly less frequently in the group receiving tacrolimus compared with the group receiving cyclosporine A [10]. When using tacrolimus in patients after kidney transplantation, diarrhea and hypomagnesemia were observed more often than in the group of patients receiving cyclosporine, but this did not have a significant effect on the outcome of transplantation. Although hemolytic uremic syndrome and thrombotic thrombocytopenic purpura can occur with the use of both cyclosporine and tacrolimus, the latter can serve as an alternative if continued immunosuppression is necessary, as cross-reactivity between drugs has not been observed [11]. The use of tacrolimus does not lead to an increase in the incidence of infections, lymphoproliferative diseases and diabetes mellitus when compared with a group of patients receiving cyclosporine A.
Interactions with drugs
Due to the fact that tacrolimus is metabolized through the cytochrome P-450 system, its kinetics may change when drugs that use the same metabolic pathways are coadministered. It should be noted that data on interactions with most drugs were obtained from in vitro experimental models and are rarely confirmed in human studies [12]. Drugs that are likely to increase the concentration of tacrolimus in the blood include: H2-histamine blockers, azole antifungals, calcium channel blockers, oral contraceptives. A decrease in the concentration of the drug is possible when taken together with antacids, rifampicin, carbamazepines, and barbiturates [8]. Despite the theoretical assumption of the possibility of an interaction between tacrolimus and glucocorticoids or methotrexate, often used in the combination treatment of graft rejection or suppression of graft-versus-host disease, a group of researchers from Seattle showed no significant difference in the clearance of tacrolimus when used together [13, 14 ]. In another study, the result of co-administration of fluconazole and tacrolimus in patients after AHSCT was a clinically insignificant decrease in the clearance of the latter by 16% [15]. However, episodes of acute renal failure have been reported in solid organ transplant patients, which may be explained by the use of a higher daily dose of tacrolimus in this group [16].
Kidney transplant
Studies have shown that the effectiveness of tacrolimus for the prevention of rejection after kidney transplantation in adults is significantly higher than cyclosporine A, even when using a microemulsion of the latter [17]. The same data were obtained when analyzing the pediatric population in prospective, randomized studies [10]. A randomized trial conducted in 50 European centers and including 560 patients after kidney transplantation showed no difference in patient survival (99.3% and 98.5%, respectively) when using tacrolimus and cyclosporine in combination with azotiaprine and steroids. However, after transplant biopsy, a difference was found in both the incidence of acute graft rejection (32.5% vs. 51.3%, p
Liver transplantation
The absence of hepatoenteric recirculation of tacrolimus and its independence from the presence of cholestasis make tacrolimus the drug of choice for liver transplantation. A meta-analysis of 717 publications (3,813 patients) in Medline showed that mortality and the risk of graft loss within the first year were significantly lower in patients receiving tacrolimus compared with all other immunosuppressive regimens (mortality rate RR 0.85, 95% Cl 0.73–0.99; graft loss: RR 0.73, 95% Cl 0.61–0.86). Tacrolimus reduces the risk of acute graft rejection (RR 0.81, 95% Cl 0.75–0.88) and steroid resistance (RR 0.54, 95% Cl 0.47–0.74). There was a higher incidence of de novo diabetes mellitus with tacrolimus (RR 0.1.38, 95% Cl 1.01–1.86), but the incidence of lymphoproliferative diseases and the need for dialysis in the development of renal failure did not differ from those in patients treated with tacrolimus. receiving cyclosporine A.
More liver transplant recipients discontinued cyclosporine A due to adverse events (RR 0.57, 95% Cl 0.49–0.66). In a study of 94 patients conducted in the USA and France, it was shown that switching from cyclosporine A to tacrolimus avoided organ rejection in 43 of 47 patients (91.5%). Their average bilirubin level decreased over 6 months from 8.7 ± 2.1 mg/dL to 2.1 ± 0.4 mg/dL (p = 0.021), and their average creatinine level decreased from 167 ± 36 μmol/L to 119 ± 28 µmol/l (p = 0.006).
Allogeneic HSC transplantation
In allogeneic HSC transplantation, the administration of calcineurin inhibitors to prevent acute graft-versus-host disease (GVHD) is the “gold standard” of immunosuppressive therapy for both related and unrelated donor transplantation. These drugs are also widely used for the treatment of chronic GVHD. In an analysis of 5 publications involving a study of 153 patients after related donor HSC transplantation, the incidence of acute GVHD was 15–44% (19, 20). Randomized trials comparing regimens using tacrolimus and cyclosporine A showed a significantly lower incidence of grade 2–4 acute GVHD in the tacrolimus group (13, 21).
A study of 329 patients with acute and chronic leukemia compared the effectiveness of prevention of acute GVHD in groups receiving tacrolimus in combination with methotrexate (n=165) and cyclosporine A in combination with methotrexate (n=164). The incidence of acute GVHD was significantly lower in the first group (31.9% versus 44.4%, p = 0.01), which made it possible to further use tacrolimus in unrelated compatible and partially compatible allogeneic HSC transplantations [22].
When analyzing transplantations from an unrelated donor, there was also a significant decrease in the incidence of grade II–IV acute GVHD in the tacrolimus group (56% vs. 74%, p
At the Scientific Research Institute of Early Childhood Education, Russian Cancer Research Center, Russian Academy of Medical Sciences, tacrolimus has been used since 2006 as a prophylaxis for acute and for the treatment of chronic GVHD during haploidentical HSC transplantations against the background of reduced-intensity conditioning regimens.
A total of 56 transplantations were performed in children with various types of solid tumors, leukemia, lymphomas and myeloproliferative syndromes. Eight patients received tacrolimus as first-line prophylaxis for acute GVHD and 4 patients as second-line for the development of cyclosporine toxicity or for the treatment of chronic GVHD. The remaining patients receiving cyclosporine formed the historical control group. Neurotoxicity and nephrotoxicity > 2 tbsp. developed in 0% versus 14% and 8% versus 20% in those who received tacrolimus and cyclosporine A, respectively. There were no significant differences in the incidence of acute GVHD above grade II. (13% vs. 22%, p > 0.05) [24].
Studies over the past decade have shown that the combination of methotrexate and tacrolimus significantly reduces the risk of developing acute GVHD compared with cyclosporine A, without increasing toxicity, infectious morbidity, or negatively affecting disease-free survival. Moreover, the use of tacrolimus in combination with mycophenolate mofetil allows us to expand the area of its use after allogeneic transplantations in patients with chronic GVHD.
The kinetics of tacrolimus in the treatment of chronic GVHD was more predictable and stable than cyclosporine A, which required less frequent determination of drug concentrations in the blood. Disadvantages include the difficulty of selecting therapeutic doses of tacrolimus in low-weight children in the absence of the drug in the form of a suspension on the Russian market.
Tacrolimus is an immunosuppressive drug that has firmly taken its place in the clinic due to its predictable kinetics, moderate toxicity and high efficiency. Due to less nephrotoxicity and lack of hepatoenteric recirculation, tacrolimus has become the drug of choice for kidney and liver transplantation compared to other calcineurin inhibitors. This drug is increasingly used every year as an alternative to cyclosporine, alone or in combination with other immunosuppressants, in programs for various types of allogeneic HSC transplantation. There is no doubt that new randomized studies are needed to determine the place of tacrolimus in transplantation of other organs and tissues.
References
- Drug monitoring and interchangeability of original and generic immunosuppressants with a narrow therapeutic index. Clinical recommendations. Russian Transplant Society, 2014. - 24 p.
- Dmitriev, R.V., Pinchuk, R.V., Storozhev, I.V. and others. The use of tacrolimus for the prevention of acute rejection of renal transplants in sensitized patients. Transplantology, 2009. - No. 2. - P. 51-53.
- VIDAL : website / Directory of medicines. - Tacrolimus, 2021. - URL: https://www.vidal.ru/drugs/molecule/1680#:~:text=Tacrolimus%20–%20highly active%20immunosuppressant., role%20in%20reaction%20rejection%20graft (date access: 06/15/2021).

![Table 3. Pharmacokinetic and pharmacodynamic parameters of fluoroquinolones with a single standard dose taken orally [7, 13]](https://irknotary.ru/wp-content/uploads/tablica-3-farmakokineticheskie-i-farmakodinamicheskie-parametry-ftorhinolonov-pri-odnokratnom-prieme-330x140.jpg)