Antidiabetic drugs are medications that lower blood glucose levels and are used to treat type 1 or type 2 diabetes.
Diabetes mellitus is a disease caused by insufficient production of insulin in the pancreas or a decrease in its function. The main symptoms of diabetes mellitus include hyperglycemia (increased blood glucose levels), glucosuria (the appearance of glucose in the urine), polyuria (increased diuresis), polydipsia (increased thirst), ketoacidosis (the appearance of toxic ketone bodies in the blood).
Insulin deficiency can be absolute or relative.
Absolute insulin deficiency develops as a result of the death of β-cells of the pancreatic islets that produce insulin. In this case, we talk about type 1 diabetes mellitus (insulin-dependent diabetes).
Type 1 diabetes mellitus usually occurs at a young age in genetically predisposed people under the influence of viruses, toxins, stress, etc. Such diabetes requires constant treatment with insulin preparations.
With a relative deficiency of insulin itself, a sufficient amount is produced, peripheral tissues are practically insensitive to it. This characterizes non-insulin-dependent diabetes or type 2 diabetes. A powerful provoking factor in the development of type 2 diabetes mellitus is obesity. The use of insulin preparations has virtually no therapeutic effect. In this case, synthetic antidiabetic agents are used.
Glucose is the main source of energy for humans. It enters the body with food as part of complex carbohydrates - polysaccharides (starch, glycogen) and disaccharides (sucrose, lactose), which, when broken down in the digestive tract, form glucose. From the small intestine, glucose is absorbed into the blood with the help of special transport proteins and distributed throughout the body.
Glucose, however, does not stay in the blood for long, quickly penetrating into the cells of the brain, muscles, liver, etc., where it is included in the biochemical processes that lead to the formation of the energy the cells need. The transport of glucose from the blood into the cells of the body's tissues is also carried out with the help of special transporter proteins, which are activated by the hormone insulin in the β-cells of the pancreatic islets.
If the pancreas produces little insulin or it is inactive against transporters, glucose cannot enter the cells, accumulating in the blood (hyperglycemia). In this case, the cell itself suffers, which, not receiving the necessary source of energy, is forced to look for other sources of energy, for example, fatty acids. However, the conversion of fatty acids occurs with the formation of toxic intermediate products - ketone bodies, which are released into the blood (ketonemia).
In the blood, ketone bodies cause damage to the walls of blood vessels and blood cells - red blood cells, platelets. Once in the brain, ketone bodies depress consciousness, impair breathing, and in large doses even cause coma.
Blood glucose, in turn, is also not a safe substance. Without getting into the cell, where it is instantly converted into energy or stored in reserve, glucose begins to oxidize blood proteins (albumin, hemoglobin) and surface proteins of the membranes of the cells of the vascular wall. This also leads to vascular damage and poor circulation.
The most sensitive to the negative effects of hyperglycemia and ketonemia are small-caliber vessels - retinal vessels, capillaries of the glomeruli of the kidneys, capillaries of the foot.
pharmachologic effect
Insulin drugs lower blood glucose levels by increasing its utilization by peripheral tissues.
In addition, insulins have an anabolic effect - they increase protein synthesis and stimulate muscle growth.
Synthetic antidiabetic drugs reduce blood glucose levels by increasing the secretion of intrinsic insulin (sulfonylurea derivatives, meglitinides, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide type 1 analogues); increasing the sensitivity of peripheral tissues to insulin (biguanides, thiazolidinediones); reducing glucose absorption in the intestine (α-glucosidase inhibitors, resins); increasing the excretion of glucose in the urine (sodium-glucose cotransporter type 2 inhibitors).
Sulfonylureas in modern clinical practice
Diabetes mellitus (DM) is the greatest non-infectious epidemic in human history. About 85–97% of DM are patients with type 2 DM (T2DM) (5). In the Russian Federation, as in all countries of the world, there is an increase in the incidence of type 2 diabetes everywhere (1, 2).
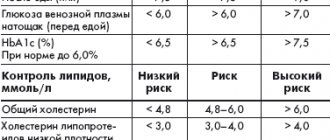
Table 1. Therapeutic goals for type 2 diabetes
Table 2. Clinical effectiveness of PSSP
Figure 1. Chemical structure of glibenclamide
Figure 2. Effect of glibenclamide on body weight compared with other PSSPs.
The scale of the problem is all the more significant, because along with officially registered cases of type 2 diabetes, a significant part of the population is not diagnosed and suffers from impaired glucose tolerance (IGT) or impaired fasting glycemia. Each year, approximately 1.5–7.3% of individuals with IGT develop type 2 diabetes (14). Consequently, the actual prevalence of type 2 diabetes is several times higher than the officially registered prevalence. It must be especially emphasized that the largest number of patients with IGT or type 2 diabetes are people of active working age.
The medical and social significance of type 2 diabetes is determined primarily by its severe complications, which lead to early disability and high mortality, a reduction in the duration and deterioration of the quality of life of patients. The development of complications is associated primarily with chronic hyperglycemia, which has been convincingly proven in long-term large-scale studies (7, 12).
Type 2 diabetes is a chronic disease that develops as a result of a combination of genetic and environmental factors (5). For type 2 diabetes, the concept of polygenic inheritance has now been accepted. Two fundamental pathophysiological mechanisms are important in the development of the disease: progressive dysfunction of pancreatic β-cells and varying degrees of insulin resistance (IR). When type 2 diabetes manifests itself, insulin secretion decreases on average by 50%, and insulin sensitivity by 70% (5). Subsequently, β-cell function deteriorates at a rate of approximately 4-5% per year from diagnosis.
IR, as the earliest disorder, is far ahead of the clinical manifestation of type 2 diabetes. This is a condition characterized by an insufficient biological response of the body to physiological concentrations of insulin. The main external factors contributing to the implementation of IR are increased consumption of high-calorie foods, insufficient physical activity and excess body weight. Under conditions of IR, which is largely due to impaired insulin action at the post-receptor level, there is a decrease in glucose utilization by muscle and adipose tissue. Liver IR is accompanied by a decrease in glycogen synthesis, activation of gluconeogenesis and glycogenolysis. Long-term IR is compensated by non-physiological hyperinsulinemia, which helps maintain normoglycemia at this stage of the disease.
In healthy people, insulin secretion in response to food intake has 2 phases. One of the early pathophysiological defects in the secretory function of β-cells in type 2 diabetes is precisely the disruption of the early phase of insulin secretion, which normally limits the non-physiological rise in glycemia in the postprandial period (5). The early phase of the prandial response, accounting for about 10% of the total daily secreted insulin, causes suppression of endogenous glucose production by the liver, suppresses glucagon secretion and lipolysis, increases the sensitivity of peripheral tissues to the action of insulin, promoting their utilization of glucose. Other features of impaired secretory function of β-cells are a reduced and time-delayed response to mixed food intake, an increase in the concentration of proinsulin, and impaired pulsatile insulin secretion.
Subsequently, the mechanism of compensatory hyperinsulinemia is lost and insulin secretion becomes insufficient in relation to increasing hyperglycemia. Under these conditions, the liver overproduces glucose, leading to fasting hyperglycemia. In addition, hepatic glucose production continues despite the nutritional load and, in combination with the relative insufficiency of insulin release, also leads to postprandial hyperglycemia. In type 2 diabetes, the greatest disproportion between insulin secretion and the need for it occurs precisely after a meal.
The results of large epidemiological and observational studies in the field of diabetes have provided convincing evidence of a close relationship between the evolution of type 2 diabetes, disturbances of carbohydrate metabolism and the increasing risk of micro- and macrovascular complications of the disease (4, 12). Thus, the UKPDS study demonstrated significant benefits of intensive glycemic control for type 2 diabetes (11): a decrease in HbA1c levels by 0.9% over a follow-up period of up to 10 years reduces the risk of death by 21%, acute myocardial infarction by 14%, and microvascular complications by 37 % and peripheral vascular diseases by 43%. It is important to note that with newly diagnosed type 2 diabetes, complications are observed in almost 50% of patients (8). Thus, according to the CODE-2 study (Cost of Diabetes in Europe - Type 2), which studied the prevalence of various chronic complications in patients with diabetes, 59% of those examined had various complications, with 23% having two, and 3% having three complications and more (7).
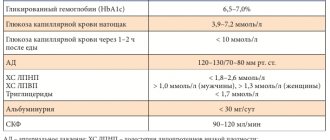
In connection with the above, optimization of approaches to therapy in patients with type 2 diabetes is a priority problem of modern medicine, and the treatment strategy should first of all prevent and minimize the risk of developing chronic complications of the disease. It is clear that a change in approaches to the treatment of type 2 diabetes is currently required to achieve more effective control of this disease. This is an intensive treatment strategy aimed against chronic hyperglycemia to achieve stable compensation of carbohydrate metabolism immediately from the moment of diagnosis of type 2 diabetes. For this purpose, many national and international diabetes associations have clearly defined the target value of the most important parameter of metabolic control - glycated hemoglobin (HbA1c): ADA/EASD:
Before moving on to the issues of pharmacotherapy for type 2 diabetes, it should be noted the importance of non-drug approaches to treating the disease and mandatory self-monitoring of glycemia, without which any pharmacotherapy will be insufficient. Modern recommendations for diet therapy for type 2 diabetes include the following principles: the energy value of food that maintains body weight close to ideal, and in case of excess weight, a low-calorie diet.
Most patients with type 2 diabetes should adhere to fractional meals (5-6 times a day in small portions), which not only avoids strong feelings of hunger while reducing the daily calorie content of portions, but also prevents post-meal hyperglycemia. The recommended content of carbohydrates is 50-60%, proteins no more than 15% of the total calorie content of the daily diet; a maximum limitation or exclusion of easily digestible carbohydrates and a predominant consumption of complex carbohydrates are provided. The share of fats in the daily diet should not exceed 30%, saturated fats should make up no more than 10% of the total fat consumed. It is envisaged to include polyunsaturated fats as an anti-atherogenic agent, it is necessary to reduce cholesterol intake (less than 300 mg per day) and increase the consumption of foods high in dietary fiber, and reduce alcohol intake (less than 30 g per day).
Simultaneously with changes in nutrition, measures should be taken to increase physical activity. Rational physical activity, safe and effective, taking into account the individual characteristics of each patient, has a good effect: for example, walking, swimming for 30-45 minutes. 3-5 times a week or any feasible set of physical exercises. Physical activity both reduces the severity of IR and stimulates the utilization of glucose by insulin-independent tissues, in which the exercise-induced increase in glucose consumption is independent of the action of insulin. During exercise, the severity of hyperinsulinemia decreases, and muscle glucose consumption increases despite a decrease in insulin levels. It is necessary to achieve weight loss if it is excess and prevent further accumulation; The safest and most reliable is a weight loss rate of 0.5-1 kg per week. The process of losing weight should be monitored by a doctor, which is especially important for elderly patients.
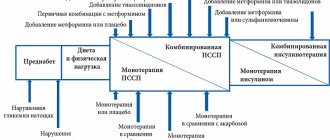
Oral hypoglycemic agents include several main groups: sulfonylureas (SMUs), biguanides, thiazolidinediones, α-glucosidase inhibitors, but recently new groups of these drugs have appeared (incretin mimetics, dipeptidyl peptidase type IV inhibitors). The initial choice of drugs remains an important task, which is helped by the analysis of the comparative effectiveness of oral glucose-lowering drugs (ALADs) (Table 2).
One of the most well-known and widely used drugs in the treatment of type 2 diabetes is PSM. The history of their creation is interesting: PSMs were developed after the side effect of hypoglycemia in laboratory animals was accidentally discovered in the 1940s while studying the antibacterial activity of sulfonamides (1). Widespread clinical use of this class of drugs began in the 50s of the last century.
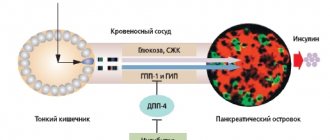
The mechanism of action of these drugs, a class of secretagogues, is primarily associated with stimulation of pancreatic β-cells, accompanied by mobilization and enhancement of endogenous insulin secretion, especially in the presence of glucose (1, 4, 6). The presence of functionally active β-cells in the islets of Langerhans is the basis for the manifestation of the effect of these glucose-lowering drugs. The peripheral (extrapancreatic) effect is apparently secondary and is caused by an increase in insulinemia and a decrease in glucose toxicity, which causes inhibition of hepatic glucose production and improvement of its utilization by peripheral tissues; its own extrapancreatic effects have not been convincingly proven (1).
Insulin is secreted by β cells in response to various stimuli, each of which contributes to the functioning of these cells. PSMs bind to specific receptor proteins on the cell membrane of β-cells, SUR-1, which are proteins of ATP-dependent K+ channels of the cell membrane (9). Currently, the role of ATP-dependent K+ channels in the regulation of insulin secretion is considered key (3, 10). After the interaction of PSM with the receptor, a chain of successive events develops: closure of ATP-dependent K+ channels for the transport of potassium ions, cessation of the transmembrane flow of potassium ions and depolarization of the membrane. Subsequently, membrane depolarization activates calcium channels, and calcium influx into β-cells increases significantly. An increase in the concentration of calcium ions inside β-cells promotes the movement of granules containing insulin across their membrane and the secretion of insulin into the bloodstream. When the level of glucose in the blood is low and the concentration of ATP inside the β-cells is low, the potassium ion transport channel is open, and due to its functioning, a membrane potential is created, which prevents the penetration of calcium ions into the β-cells, which are necessary for the movement of granules containing insulin through the membrane β-cells and hormone secretion into the bloodstream. Therefore, PSMs activate the physiological mechanism by which glucose stimulates insulin production.
The principle approach to choosing a particular PSM is based on assessing the balance of effectiveness, safety and accessibility for the patient. The unequal affinity of PSM for specific receptors of pancreatic β-cells determines their different glucose-lowering activity (6, 4). The higher the affinity of the drug for the receptor, the longer its inhibitory effect on ATP-dependent K+ channels will be, and therefore, the more endogenous insulin secretion will be stimulated due to the entry of calcium ions into β-cells. Among PSMs, glibenclamide has the most pronounced hypoglycemic effect, since the drug is characterized by maximum affinity for ATP-dependent K+ channels of β-cells. Moreover, the high glucose-lowering activity of the drug is also explained by the peculiarities of the chemical structure - the presence of not only a sulfonylurea, but also a benzamide group (Figure 1). So, interacting with two binding sites of pancreatic β-cell receptors, glibenclamide most quickly and strongly promotes the closure of ATP-dependent K+ channels, stimulates membrane depolarization, an increase in intracellular Ca2+ and, consequently, the secretion of endogenous insulin.
Currently, glibenclamide is the only PSM whose therapy has been proven to reduce the risk of developing chronic complications of type 2 diabetes. It is significant that, according to the URPDS study, treatment with glibenclamide reduced the risk of developing microvascular complications by 30% (p = 0.015), including retinopathy requiring photocoagulation – by 33% (p = 0.008). The problem of macrovascular complications of type 2 diabetes seems even more serious: glibenclamide significantly reduced the incidence of infarction by 22% (p = 0.056). Due to such a pronounced glucose-lowering effect documented in the URPDS and long-term experience with use, which distinguishes glibenclamide from all other SLMs, this drug remains the gold standard of oral glucose-lowering therapy and the most commonly used SLM (13).
In the Russian Federation, both the traditional form of glibenclamide (non-micronized, 5 mg) and micronized forms of the drug are used. Due to the peculiarities of pharmacokinetics and pharmacodynamics, micronized forms of glibenclamide (drug Maninil®, Germany) (1.75/3.5 mg) are currently prescribed more widely than non-micronized forms. The great advantage of the micronized form of glibenclamide is its rapid absorption (complete release of the active substance within 5 minutes after dissolution) and, accordingly, complete bioavailability (100%), due to which the daily dose of glibenclamide is reduced by 30-40% in contrast to the traditional form of glibenclamide 5 mg. The therapeutic concentration of the drug Maninil® in the blood is achieved quickly, within 15-30 minutes. The half-life of the micronized form of glibenclamide is 1.5-3.5 hours; The duration of the hypoglycemic effect, however, does not correspond to this period and amounts to a day. Thanks to these pharmacokinetic characteristics, micronized forms of glibenclamide can be taken 1-2 times a day, which is convenient for patients, and the smooth achievement of maximum concentration after 2.5 hours - on the rise of postprandial hyperglycemia - avoids the risk of hypoglycemia between meals, which, without a doubt, important for elderly patients.
The initial dose of the micronized form of glibenclamide is usually 1.75-3.5 mg per day; Maninil® must be taken immediately before meals. The dose of the drug is gradually titrated until the required therapeutic effect is achieved - target glycemic indicators - no faster than every 5-7 days; if the daily dose is 50% of the maximum therapeutic dose (14 mg/day), then a 2-fold dose of Maninil is recommended.
Glibenclamide is metabolized to form inactive and active oxymetabolites and is characterized by a dual excretion route: 50% through the kidneys, the same amount with bile. In chronic renal failure, the excretion of glibenclamide does not change, but protein binding decreases, resulting in an increase in the free fraction of Maninil and the risk of hypoglycemia (1).
You should always remember about the possible risk of developing hypoglycemic conditions, especially dangerous in old age, during glucose-lowering therapy. One of the common causes of hypoglycemia is a rapid increase in the daily dose or incorrectly selected doses of Maninil, as well as a violation of the diet (sharp restriction of complex carbohydrates, skipping the next meal) and irrational physical activity.
Patients with type 2 diabetes often have various comorbidities, so in clinical practice it is necessary to take into account the problem of drug interactions. Thus, when prescribing a number of drugs due to various mechanisms, both an increase and decrease in the effect of glibenclamide, as well as other PSMs, can be observed: glucocorticoids, barbiturates, phenothiazines, thiazide diuretics, thyroid hormones (suppressive therapy), estrogens, gestagens, adrenomimetics, nicotinic acid derivatives, rifampicin. On the contrary, the following drugs enhance the hypoglycemic effect: salicylates, sulfonamides, anabolic steroids, pentoxifylline, allopurinol, chloramphenicol, pyrazolone derivatives, clofibrate, bezafibrate, monoamine oxidase inhibitors, indirect anticoagulants, systemic antifungals, alcohol-containing drugs.
It is known that 85-90% of patients with type 2 diabetes are overweight or obese. An undesirable side effect of glibenclamide, like other PSMs, in some cases may be the progression of obesity (Figure 2), which can be reduced or prevented by following medical recommendations on nutrition and physical activity, which is often not actively put into practice by patients.
It is impossible to ignore such an important issue as the availability of therapy, due to both the chronic nature of the disease and the need for long-term glucose-lowering therapy, as well as the huge, constantly growing number of patients with type 2 diabetes and the difficult economic situation in our country. In this regard, the most advantageous is the use of Maninil, taking into account the high efficiency, long-term experience of use and the availability of a modern micronized form of the drug. WHO experts also emphasize the high social significance of glibenclamide, having once again included it in the list of Essential Medicines - the most effective, safe and beneficial from a pharmaco-economic point of view for the treatment of socially significant diseases; Glibenclamide is the only PSM representative on this list (15).
Contraindications to the use of Maninil are: type 1 diabetes, diabetes after pancreatectomy, ketoacidosis, surgical interventions (major operations), severe infections and injuries, a history of allergies to PSM or similar drugs, severe renal and liver dysfunction. You should refrain from prescribing the drug during pregnancy and lactation.
In the majority of patients with type 2 diabetes, monotherapy with PSSP does not provide long-term effective glycemic control: according to the results of the UKPDS study, monotherapy with one of the oral hypoglycemic drugs after 3 years from the start of treatment was effective in only half of the patients, and after 9 years - in only 25%; this leads to the need for combination therapy (11). Further maintenance of compensation of carbohydrate metabolism can be achieved by using a combination of two or three PSSPs with different mechanisms of action or by adding basal insulin to treatment. Maninil® can be effectively used in combination therapy with metformin, thiazolidinediones, incretin mimetics, and insulin (4, 6).
In conclusion, it should be noted that when prescribing hypoglycemic therapy, one should not neglect PSM - the basis of pharmacotherapy of the disease - Maninil with long-term experience in clinical use and proven effectiveness, which will delay or delay the onset of complications of the disease, improve the prognosis and improve the quality of life of patients.
Classification of antidiabetic drugs
Insulin preparations are classified by origin into bovine (beef), pork and human insulin.
Bovine and porcine insulins are obtained from the pancreas of cattle and pigs, respectively.
Human (recombinant, genetically engineered) insulin is produced using biotechnology - the human insulin gene is introduced into the cell of baker's yeast or E. coli, which then begins to produce a hormone completely similar to human insulin.
In addition, insulin preparations are classified according to their duration of action:
- fast-acting insulin preparations (onset of action - 15-30 minutes, duration - 4-6 hours): insulin lispro, insulin aspart, insulin glulisine;
- medium-acting insulin preparations (onset of action – 1-1.5 hours, duration – 8-12 hours): human insulin, pork insulin;
- long-acting insulin preparations (onset of action – 4-8 hours min, duration – 20-30 hours): insulin glargine, insulin detemir, insulin degludec.
In addition, there are combination preparations of long-acting insulin with fast-acting insulin (insulin degludec + insulin aspart), as well as a combination of long-acting insulin with a glucagon-like peptide-1 agonist (insulin glargine + lixisenatide).
Synthetic antidiabetic drugs are classified according to their chemical structure, mechanism of action and origin:
- sulfonylurea derivatives: glibenclamide, gliclazide, glimepiride, gliquidone;
- biguanides: metformin;
- thiazolidinediones: pioglitazone, rosiglitazone;
- α-glucosidase inhibitors: acarbose, voglibose;
- dipeptidyl peptidase-4 inhibitors: sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin, gemigliptin;
- glucagon-like peptide type 1 analogues: exenatide, liraglutide, lixisenatide, dulaglutide;
- sodium-glucose cotransporter type 2 inhibitors: dapagliflozin, canagliflozin, empagliflozin;
- meglitinides: nateglinide, repaglinide;
- aldose reductase inhibitors: isodibut;
- gums: guar gum;
- preparations of plant origin: blueberry shoots, bean fruit leaves.
In addition, in cases where one drug is not enough to achieve a pronounced hypoglycemic effect, effective combinations have been developed: biguanides + sulfonylurea derivatives; biguanides + dipeptidyl peptidase-4 inhibitors; thiazolidinediones + dipeptidyl peptidase-4 inhibitors; sodium-glucose cotransporter type 2 inhibitors + dipeptidyl peptidase-4 inhibitors; biguanides + sulfonylurea derivatives + thiazolidinediones.
Change and intensification of therapy
At the first or subsequent visits, the patient should be warned about the possible need to intensify therapy:
“Over time, the disease usually progresses and the effect of the drug weakens. If one tablet drug is insufficiently effective, it may be necessary to increase the dose and/or add a second and sometimes a third drug. The time it takes for the effect of therapy to decrease is very individual. Insulin therapy may be required in the future to control blood sugar levels. It can be temporary (for example, during surgery) or prescribed permanently. If this happens, it will not be your fault.”
It may be noted that in some cases, oral therapy may be temporarily withheld when good control is achieved - most often when there is a significant decrease in body weight. But in most cases, they switch from taking one drug to taking another tablet drug (combination). When intensifying therapy, you can use a more convenient option of taking two drugs at once in one tablet (fixed combinations). The possibility of using fixed combinations is important for patients with T2DM, since the disease is often accompanied by concomitant pathology and, accordingly, the use of other drugs. The patient should be sufficiently aware of the benefits of fixed combinations, since this may increase his adherence to treatment. It is worth dwelling on the most common types of combination therapy:
- Metformin + PSM is a combination that allows you to increase insulin production and sensitivity to it in the body. Rapidly lowers blood sugar levels, but has disadvantages regarding the risk of hypoglycemia and weight gain;
- Metformin + DPP-4 inhibitors - a combination that allows you to increase insulin production through the glucose-dependent pathway and insulin sensitivity. Does not lead to the development of hypoglycemia and weight gain.
Basics of diabetes treatment
Antidiabetic drugs are selected individually by an experienced endocrinologist, taking into account the patient’s body weight, type and severity of diabetes mellitus, and concomitant diseases.
Patients with type 1 diabetes mellitus use insulin preparations for life.
In the treatment of type 1 diabetes, in addition to insulin medications, a low-carbohydrate diet is important. To control carbohydrate consumption, as well as calculate the individual dose of insulin, use the system of bread units - XE, where 1 XE is equal to 1 piece of white or rye bread.
The use of insulin preparations should imitate the rhythm of natural insulin secretion, for which insulin preparations of different durations of action are used. Typically, long-acting insulin is used in the morning, and short-acting insulin is used three times a day, 15-20 minutes before meals. Skipping meals is strictly not recommended.
Insulin preparations are administered subcutaneously using a disposable plastic syringe or syringe pen with replaceable cartridges. Patients need to have 2 syringe pens - one for short-acting insulin, the other for long-acting insulin.
In emergency cases (hyperglycemic coma), intravenous administration of short-acting insulin is acceptable.
In the treatment of type 2 diabetes, in addition to medications, diet and regular moderate exercise are important.
The effect of drugs of this pharmacological group is maintained only with regular use, therefore synthetic antidiabetic drugs are taken constantly for a long time (most often for life).
Almost all synthetic antidiabetic drugs are used orally. The exception is type 1 glucagon-like peptide analogues, which are administered subcutaneously.
Control issues
Young patients should be warned that good metabolic control helps prevent long-term complications. To monitor the course of the disease, it is necessary to conduct a regular medical examination, including assessment of the condition of the fundus, assessment of pulsation in the arteries of the feet, sensitivity of the feet, determination of a number of laboratory indicators (levels of glycated hemoglobin, lipids, creatinine, glomerular filtration rate, etc.).
Self-monitoring is a good way to actively involve the patient in the treatment process. It includes self-measurement of blood glucose levels, monitoring of body weight, self-measurement of blood pressure, and daily foot examination. The frequency of self-measurement of blood glucose during the treatment of PSSP is determined individually and depends on the initial level of glycemic control, but at least 1-2 times a week before and 2 hours after meals. It is necessary to notify the patient at what glycemic levels he needs to see a doctor.
For older patients, self-monitoring should focus on the absence of symptoms of high blood sugar. Self-administered tests may be useful if you feel unwell.
It is important to note that blood sugar and glycated hemoglobin levels will be regularly assessed during visits to the doctor and reflect the results of the patient's efforts.