Metformin in the treatment of type 2 diabetes mellitus: a bestseller not fully read
Metformin is one of the “oldest” drugs in the treatment of type 2 diabetes mellitus (type 2 DM), first synthesized in 1922 and began to be used in clinical practice since the late 50s. last century. Metformin is currently the only representative of the biguanide group used for the treatment of type 2 diabetes.
Mechanism of action
In 2001, the main mechanism of action of the drug was established - activation of 5'AMP-activated protein kinase (AMPK, AMP activated protein kinase) - a cellular kinase that controls the energy balance of the cell through a direct effect on gene transcription and key metabolic enzymes. AMPK is found in the cell nucleus and cytoplasm: nuclear AMPK is directly capable of regulating gene expression; cytosolic AMPK regulates the functions of cytosolic and membrane proteins. Fluctuations in the level of AMP:ATP in the cell can act as a kind of sensory signal for changes in AMPK activity. The study of the integrative role of AMPK as a regulator of energy metabolism is still ongoing. It has now been established that AMPK is involved in many physiological processes. Activation of AMPK during contraction of muscle fibers (AMPK activity of myocytes is also regulated by resistin, TNF-alpha, leptin, adiponectin) mediates the positive metabolic and cardiovascular effects of physical training. Hypothalamic AMPK is involved in the regulation of appetite (AMPK activity is in turn regulated by leptin, ghrelin, insulin, adiponectin) and, possibly, circadian rhythms. AMPK is a regulator of fatty acid and glucose metabolism: activation of AMPK stimulates the oxidation of fatty acids in the liver and ketogenesis, suppresses lipogenesis, the synthesis of cholesterol and triglycerides, suppresses lipolysis and lipogenesis in adipocytes, stimulates the oxidation of fatty acids and glucose uptake in skeletal muscles through increased protein biosynthesis - glucose transporters, modulates insulin secretion by beta cells. It has been suggested that AMPK dysregulation (decreased expression and activity) is associated with the development of obesity, prediabetes/DM, metabolic syndrome, cancer, myocardial ischemia, myocardial ischemia-reperfusion injury, and possibly myocardial hypertrophy [1].
How metformin activates AMPK is not completely clear. One possible mechanism may be the damaging effect of metformin on the I-complex of mitochondria (NADH-ubiquinone oxidoreductase - the initial component of the respiratory chain), which leads to an increase in the level of AMP in the cytosol and thereby triggers the natural pathway of AMPK activation. The AMPK-dependent mechanism of action of metformin as an antidiabetic drug is realized by suppressing the expression of genes responsible for gluconeogenesis and lipogenesis; accordingly, glucose production in the liver decreases, fatty acid oxidation increases, insulin sensitivity and peripheral glucose uptake increases, and glucose absorption in the intestine decreases [1 ]. The activation of AMPK is also associated with the pleiotropic effects of the drug, which determine its advantages among antidiabetic drugs.
Pleiotropic effects
Metformin is an insulin sensitizer that has a pronounced antidiabetic effect (reduction in HbA1c by 1.0-2.0%), not accompanied by a significant increase in the risk of hypoglycemia (hypoglycemia is possible with intense physical activity, a low-calorie diet, or combination with other antidiabetic drugs); at least does not promote weight gain or moderately reduces it; moderately reduces the level of triglycerides and low-density lipoproteins, is economical [2, 3] and, in addition, has additional benefits, some of which have been widely discussed recently.
- According to one of the most cited studies of the last century, UKPDS, metformin, having demonstrated similar effectiveness to sulfonylureas in controlling blood glucose levels, showed the ability to provide cardiovascular protection (reducing the risk of myocardial infarction by 39%) and increasing patient survival compared with sulfonylureas or insulin [4]. UKPDS was the first study to show improved clinical outcomes with metformin therapy and prompted a more in-depth study of the drug's effects, confirming the first optimistic results. Partly, the metabolic and protective effect of the drug in the myocardium is AMPK-independent and is realized with the participation of the protein kinase C family and the p38 mitogen-activated protein kinase family: p38 MAPK (p38 mitogen-activated protein kinases)-dependent and PKC protein kinase C)-dependent mechanism [5 ].
- Metformin reduces the increased risk of developing certain forms of cancer in type 2 diabetes (pancreas, ovaries, mammary glands, intestines, lungs) [6–9]. Presumably, the antiproliferative and antiangiogenetic effect of the drug is associated with the activation of AMPK, the effect on the G0-G1 phase of the cell cycle, and interaction with transmembrane receptors of the G protein-coupled receptors family [7].
- Metformin has shown an osteogenic effect in vitro and in vivo, a protective effect against osteoporotic bone fractures, and a theoretically promising preventive potential against the increased risk of osteoporosis in diabetes [10–12]. Presumably, the osteogenic effect of metformin is realized by influencing the activity of AMPK and the osteoblast-specific transcription factor Runx2/Cbfa1 [11].
Destination Features
Based on the results of numerous studies and many years of worldwide clinical experience with its use, metformin has become a clear dominant drug in the treatment of type 2 diabetes. A recently published study was the first to prove the advisability of starting metformin therapy as early as possible in type 2 diabetes [13]. In a group of 1799 patients with type 2 diabetes, clinical successes were analyzed at different periods of starting therapy from the moment of diagnosis of the disease: 40% of patients whose first-line drug was metformin began taking it in the next 3 months; 25% - after 3 months or more; 27% started taking metformin with HbA1c < 7%; 23% - only after HbA1c became above 9%. The researchers considered an increase in HbA1c > 7.5% or the need to prescribe other antidiabetic agents in the form of addition to or replacement of metformin as secondary treatment failure. Overall, 42% of secondary failures were recorded during follow-up (17%/year). At the same time, the lowest rate of treatment failure was observed among patients who began treatment with metformin up to 3 months after the diagnosis of diabetes (12.2%/year), and patients who began drug therapy with an HbA1c level < 7% (12.3%/year). year). In cases where the start of therapy was delayed by 4-11 months, treatment failure was 56% more likely (OR 1.56; 95% CI 1.12-2.18), and in cases of treatment failure by three or more years, the probability of failure was the highest (OR 2.20; 95% CI 1.68–2.87).
Today, according to the recommendations of the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD), metformin is positioned as the first choice for the treatment of type 2 diabetes [2], a pathogenetic agent that is safely and effectively combined with most drugs used in Currently, antidiabetic drugs are a drug that should appear in the life of a patient with type 2 diabetes as early as possible and can accompany him for as long as possible.
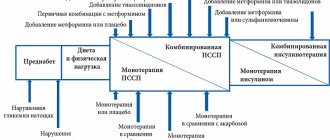
The accumulated data on the safety profile have now made it possible to quite clearly define the boundaries of the possible use of the drug in pathology of the kidneys and liver, and to raise the age threshold for use to the age of 80 years (and older, subject to the following conditions) (
).
Currently, metformin is one of the most effective and safe drugs. However, in everyday clinical practice there may be problems associated with the choice of antidiabetic therapy, and the priority of metformin may not seem so clear. When discussing the use of metformin among patients with type 2 diabetes, there are at least three “special groups” that the practitioner encounters most often.
Patients with type 2 diabetes without overweight and obesity
Metformin is the first choice drug in the treatment of type 2 diabetes without specifying such initial patient characteristics as body weight. At the same time, the vast majority of studies of cohorts of patients with type 2 diabetes, which created the reputation of metformin as a “best seller,” included patients with overweight and/or obesity. Of course, it should be remembered that in the group of patients with hyperglycemia and no excess body weight, first of all, type 1 diabetes (LADA), chronic pancreatitis, pancreatic cancer and other causes of diabetes should be excluded. That is, in this group of patients, type 2 diabetes is, in a certain sense, a diagnosis of exclusion. In the context of the known possible reduction in body weight against the background of metformin, as well as the essence of its antidiabetic action (reduction of insulin resistance characteristic of overweight/obesity), the question naturally arises about the advisability of using the drug in a small group of patients with type 2 diabetes without increasing body weight.
Short-term (3-12 months) studies previously demonstrated the effectiveness of the drug in a group of patients without obesity: no difference in the reduction of HbA1c between patients with excess body weight (> 25 kg/m2) and patients with a body mass index (BMI) < 25 kg /m2 (including those with BMI < 22 kg/m2); greater efficiency compared to acarbose (metformin 500-750 mg/day vs acarbose 150-300 mg/day); equivalent efficacy when compared with repaglinide [16–22].
Of particular note is a recent retrospective study of the long-term (3-year follow-up) effects of metformin in a group of patients with BMI < 25 kg/m2 (n = 108) and BMI > 25 kg/m2 (n = 105) [23]. During the study, the rate of decrease in HbA1c slowed down in both groups, reflecting a natural decrease in beta-cell function, the daily dose of metformin required for effective control of blood glucose levels increased, however, in the group of patients without excess body weight, the need for metformin by the end of the observation period was significantly less (metformin doses 677 ± 184 mg/day and 724 ± 117 mg/day, respectively), but turned out to be higher when calculated based on the patients’ body weight. The explanation for this fact is given by the researchers themselves: the group of patients without excess body weight had a longer duration of diabetes, a higher frequency of use of other antidiabetic drugs, a lower percentage of visceral fat, which indirectly indicates a more pronounced defect in insulin secretion and, accordingly, a smaller contribution of insulin resistance to development Type 2 diabetes. It should be noted that the BMI value did not change significantly during observation in both groups. Thus, in the group with a BMI < 25 kg/m2, against the background of significantly lower absolute doses of metformin, no undesirable weight loss was noted in this case.
This observation, of course, is not without the shortcomings of all retrospective observations, however, it once again demonstrated that the use of metformin in patients without excess body weight is justified by its effectiveness and good tolerability during long-term observation [24]. Theoretically, in a group of patients without overweight/obesity, metformin should also retain its pleiotropic, non-antidiabetic effects. Current research cannot answer this question; further study of this aspect of the drug’s action is necessary.
Patients with type 2 diabetes and non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) includes non-alcoholic steatosis and non-alcoholic steatohepatitis with or without fibrosis, liver cirrhosis [24]. NAFLD affects 10–39% of the general population, 50% of patients with diabetes, 57–74% of obese patients, and up to 90% of patients with morbid obesity [25].
Severe insulin resistance, which accompanies the vast majority of patients with type 2 diabetes, is a removable factor predisposing to the development of liver damage [26–28]. In this regard, the use of metformin as a drug that increases insulin sensitivity is pathogenetically justified. Moreover, the liver is the primary target organ for the action of metformin; AMPK is a key mediator of the effect of metformin on gluconeogenesis and lipogenesis in the liver. AMPK-dependent suppression of lipogenic enzymes (primarily acetyl-CoA carboxylase), a decrease in plasma triglyceride levels and a decrease in the intracellular lipid content in hepatocytes can prevent static hepatosis, as demonstrated in experimental studies [29, 30].
Individual, usually few, clinical studies demonstrate the positive effects of metformin in groups of patients with NAFLD [31–35]. However, according to a meta-analysis by Rakoski MO et al. (2010), metformin did not show significant histological and biochemical success in the group of patients with non-alcoholic steatohepatitis with type 2 diabetes and without type 2 diabetes, in contrast to glitazones [36]. However, to date, the final point in the confrontation between these two classes of drugs that affect insulin resistance and have potential applications in NAFLD has not been set, largely due to the lack of properly designed randomized trials of sufficient size and duration with adequately selected endpoints. points (primarily histological) [37].
It should be remembered that if there are signs of active liver disease of any etiology (ALT exceeds the upper limit of normal by > 2.5 times, > 7 points on the Child-Pugh scale), the drug should not be used [14].
Patients with type 2 diabetes and heart failure
Diabetes mellitus is an independent risk factor for the development of heart failure (HF): the risk is 2 times higher in men and 5 times higher in women with diabetes compared to the general population [38, 39]. The risk of developing HF increases by 8–12% for every 1% increase in HbA1c [40, 41]. On the other hand, patients with chronic HF have an increased risk of developing diabetes [42]. As a result, HF is present in 20–25% of adult patients with diabetes, and patients with diabetes have a 2-fold higher risk of hospitalization and death due to HF [40]. Current clinical practice is such that, according to some data, up to 24.5% of patients with type 2 diabetes receive metformin, having certain contraindications to it [43]. The actual incidence of metformin use in HF is unknown; according to an analysis of published studies (Eurich DT et al., 2007), 10% to 25% of patients receiving metformin for the treatment of type 2 diabetes have HF [44].
Turning to the current contraindications to the use of metformin, it should be noted that heart failure, requiring pharmacological therapy among other causes of hypoxia, has been identified as a risk factor for the development of lactic acidosis while taking the drug. Lactic acidosis is registered during metformin therapy in 6.3 cases per 100,000 patients/year [45]. According to the results of an analysis conducted by a representative of the Food and Drug Administration (USA) Misbin RI and published in 2004, the following can be highlighted: the increased risk of lactic acidosis when using metformin in the absence of a contraindication approaches zero; metformin is associated with lactic acidosis in patients with reasons for its development (HF, hypoxia, sepsis, etc.); metformin can be an independent cause of lactic acidosis in case of overdose; One may be concerned about the development of lactic acidosis when the drug accumulates in the case of renal failure [46]. It was the “ghost” of lactic acidosis, hovering since the use of phenformin, and the lack of large, purposefully designed studies that until recently significantly limited the prospects for the use of metformin in patients with HF, i.e. in every fourth patient with diabetes.
In the group of patients under consideration, the difference between hypoxia against the background of class II and IV heart failure is intuitively clear, as is the difference in the functional state of the kidneys in these cases, which also implies different degrees of risk of lactic acidosis associated with hypoxia. However, no prospective study has yet made it possible to stratify HF in patients with type 2 diabetes according to the risk of developing lactic acidosis during the use of metformin. Of course, one of the main reasons for this is the rarity of the condition being studied when using the drug.
Retrospective cohort studies indicate that cases of lactic acidosis are rare events, even in subgroups of patients who have contraindications to the use of the drug [43, 46]. However, age, acute renal failure, acute cardiorespiratory pathology, sepsis due to diabetes, and drug overdose remain important as risk factors for lactic acidosis [47–50]. However, metformin may even play a protective role in cases of severe lactic acidosis not associated with medication [51].
Data are accumulating on the safety and possible additional benefit of metformin in patients with stable chronic HF, based on the HF ⇔ cardiac insulin resistance theory [52, 53]. Thus, according to a systematic review and meta-analysis by Eurich DT et al. (2007) metformin therapy in patients with diabetes and heart failure is associated with a reduction in all hospitalizations compared with other antidiabetic drugs (OR 0.85, 0.76-0.95; I2 = 21%; p = 0.004), with a significant reduction in mortality from all causes [44]. In a prospective case-control study (UK General Practice Research Database cohort, patients with diabetes and heart failure, n = 8,404), the results of which were published in 2010, the use of metformin as monotherapy versus other antidiabetic drugs was compared with no Any antidiabetic therapy was associated with a lower mortality rate (OR 0.65, 0.48–0.87 in the metformin group and OR 0.72, 0.59–0.90 in the no antidiabetic therapy group) [53]. In a cohort prospective 2-year study of more than 6 thousand patients with diabetes and CH Aguilar D. et al. (2011) also showed that metformin is associated with a lower mortality rate in patients with diabetes and heart failure (15.8% vs 25.5%, p < 0.001) [54].
The cardioprotective effect of metformin in heart failure cannot be explained solely by its antidiabetic effect; it may be related to the described effects of the drug on lipid metabolism, endothelial function, cardiomyocytes and vascular smooth muscle cells, platelet activity and blood coagulation [55]. The pleiotropic effects of metformin are mediated in part by its primary role as an AMPK activator. Experimental work demonstrates that preventing the progression of HF and increasing survival in HF, including ischemia-induced, may be associated with the drug’s action as an AMPK activator, which leads to an increase in the activity of nitric oxide synthetase (eNOS) and the expression of a transcriptional coactivator and metabolic regulator - coactivator Peroxisome proliferator-activated receptor gamma 1alpha (PGC-alpha), which plays an important role in regulating mitochondrial biogenesis and function. This leads to an increase in plasma NO levels, increased insulin sensitivity and, as a consequence, suppression of cardiomyocyte apoptosis and improvement of myocardial function [55, 56]. In addition, metformin inhibits collagen synthesis in vitro (in cultured cardiac fibroblasts) and suppresses the development of cardiac fibrosis induced by pressure overload in vivo through inhibition of the TGF-beta-Smad3 signaling pathway [57].
In 2006, the FDA approved a change in the drug label to list HF as a condition for which precautions should be taken rather than as a direct contraindication to the use of metformin [50]. Thus, today metformin is contraindicated:
1) for kidney disease or impaired renal function (creatinine level > 1.4 mg/dl for women and > 1.5 mg/dl for men), which may result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, sepsis; 2) with known hypersensitivity to the drug; 3) in acute or chronic metabolic acidosis, including diabetic ketoacidosis (with or without coma) [50].
In addition, you should be aware of situations that require temporary discontinuation of the drug in both patients with and without HF:
- When planning a radiological examination using intravenous iodine-containing contrast agents (iv urography, cholangiography, angiography, CT with intravenous contrast) due to possible acute impairment of renal function and, accordingly, an increased risk of lactic acidosis, metformin should be temporarily discontinued before 48 days. hours before the procedure and for the next 48 hours after it, with resumption of use after confirmation of the preservation of normal renal function.
- When planning any surgical interventions, metformin should be temporarily discontinued (with the exception of minor interventions without stopping food and water intake), the drug is resumed with the resumption of food intake, provided that normal renal function is maintained [50].
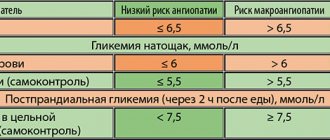
Metformin - a vascular drug with antidiabetic effect?
Experimental and clinical work in recent years demonstrate that the protective pleiotropic effects of metformin are found not only in HF [58, 59]. Presumably, the protective activity of the drug may be based on a wide range of effects, opening up attractive clinical prospects (
).
With regard to the cardioprotective effect of metformin in acute myocardial ischemia, the step from experimental work with positive results [60] to clinical practice is enormous. In the situation of the development of acute coronary syndrome (ACS) in a real patient with type 2 diabetes, the benefit of using metformin in presumably reducing the area of ischemic damage and limiting the extent of myocardial necrosis should outweigh the known concomitant risk of developing lactic acidosis due to hypoxia and renal dysfunction. Of course, given how the clinical “fate” of metformin is developing, perhaps the balance of “benefit/harm” of using the drug in a given clinical situation will tip in the direction of benefit, but one thing is certain - only large randomized clinical trials will be able to answer this question. In the absence of a proper evidence base for safety in the situation of the development of ACS, as well as acute HF, Metformine should not be used: “guilty until proven otherwise.”
In contrast to myocardial ischemia, it became possible to discuss the recently published results of a large clinical trial of the safety and efficacy of metformin in patients with ischemic lesions of the lower extremities and type 2 diabetes [61]. In a cohort of patients with atherothrombosis (group of patients receiving metformin, n = 7,457 vs group of patients not receiving metformin, n = 12,234), it was demonstrated that during a 2-year follow-up, metformin significantly reduced the risk of all-cause mortality by 24 % (p < 0.001). Thus, there is another argument in favor of using the drug in high-risk patients with type 2 diabetes and concomitant cardiovascular pathology, based not only on safety evidence, but also on evidence of additional benefits of use.
Thus, today metformin is a real bestseller in clinical practice for the treatment of type 2 diabetes, a rare case when, with increasing experience of use, new advantages rather than disadvantages of the drug are discovered. Studies of the last decade, devoted to various aspects of the use of metformin, demonstrate the unique uniqueness of the drug - an still inexhaustible therapeutic potential, the boundaries of which tend to expand.
Literature
- Steinberg GR, Kemp BE AMPK in Health and Disease // Physiol Rev. 2009. 89: 1025–1078.
- Nathan DM, Buse JB, Davidson MB et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes // Diabetologia. 2009, 52: 17–30.
- Bolen S., Feldman L., Vassy J. et al. Systematic review: comparative effectiveness and safety of oral medication for type 2 diabetes mellitus Ann Intern Med. 2007; 147 (6): 386–399.
- UK Prospective Diabetes Study (UKPDS) Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) // Lancet. 1998, 352: 854–865.
- Saeedi R, Parsons HL, Wambolt RB et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms // Am J Physiol Heart Circ Physiol. 2008; 294(6):H2497–506.
- Bowker SL, Majumdar SR, Veugelers P. et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin // Diabetes Care. 2006; 29: 254–258.
- Shawn M., Cripps R. Diabetes Mellitus and Increased Risk of Cancer: Focus on Metformin and the Insulin Analogues // Pharmacotherapy. 2010; 30 (11): 1159–1178.
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD Metformin and reduced risk of cancer of 25–37% in diabetic patients // BMJ. 2005; 330: 1304–1305.
- Monami M., Colombi C., Balzi D. et al. Metformin and Cancer Occurrence in Insulin-Treated Type 2 Diabetic Patients // Diabetes Care January. 2011. 34: 129–131.
- Vestergaard P., Rejnmark L., Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk // Diabetologia. 2005. 48: 1292–1299.
- Molinuevo S. Schurman L., McCarthy AD et al. Effect of metformin on bone marrow progenitor cell differentiation: In vivo and in vitro studies // Journal of Bone and Mineral Research. 2010; V.25: 211-221.
- Khazai NB, Beck G. R Jr., Umpierrez GE Diabetes and fractures: an overshadowed association // Endocrinology, Diabetes & Obesity: 2009; V. 16. Iss 6: 435-445.
- Larson NF Metformin More Effective if Initiated Soon After Diabetes Diagnosis // Diabetes Care. 2010; 33: 501–506.
- Mazze RS, Strock E., Simonson GD, Bergenstal RM Prevention, diagnosis and treatment of diabetes mellitus in adults. 4th ed., revision. Park Nicollet, 2007.
- Rational pharmacotherapy of diseases of the endocrine system and metabolic disorders: Hand. for practicing doctors/Under general. ed. I. I. Dedova, G. A. Melnichenko. M.: Litterra, 2006. 1080 p.
- Kaku K., Tajima N., Kawamori K. Melbin Observation Research (MORE) study of metformin therapy in patients with type 2 diabetes mellitus // J Japan Diab Soc. 2006, 49: 325–331.
- Hosokawa K., Meguro S., Funae O. et al. Clinical effects of metformin with nonobese type 2 diabetes // J Japan Diab Soc. 2009, 52: 1-6.
- Clarke BF, Campbell IW Comparison of metformin and chlorpropamide in non-obese, maturity-onset diabetics uncontrolled by diet // Br Med J. 1977, 2 (6102): 1576-1578.
- Yajima K., Shimada A., Hirose H., Kasuga A., Saruta T. “Low dose” metformin improves hyperglycemia better than acarbose in type 2 diabetics // Rev Diabet Stud. 2004, 1: 89–94.
- Lund SS, Tarnow L, Stehouwer CD et al. Targeting hyperglycaemia with either metformin or repaglinide in non-obese patients with type 2 diabetes: results from a randomized crossover trial // Diabetes Obes Metab. 2007, 9: 394–407.
For the rest of the bibliography, please contact the editor.
I. V. Druk, Candidate of Medical Sciences, Associate Professor Omsk State Medical Academy, Omsk
Contact information about the author for correspondence
Table 1. Table 2. [59]
Rules for writing a prescription
Metformin is dispensed at pharmacies, subject to a prescription. The Latin prescription for Metformin must be written correctly. If there are errors in the document, it will be rejected by the pharmacist.
In Latin, the prescription for Metformin allows you to prescribe the form of the drug, dosage, and rules of administration. The document begins with the word Recipe (take). It is written on a new line. The word Recipe can be abbreviated as follows: Rp. This designation must be followed by a period and a colon (Rp.:).
After the word Recipe, write the name of the drug in Latin, the dosage and form of the medicine. In this case, the doctor will write the following: “Tabulettas Metformini 1.0.” The word Tabulettas can be abbreviated as Tab.
After describing the dose, form and name of the medicine, the doctor prescribes in the second line of the prescription the number of forms that the pharmacist must dispense. Example: “Da tales doses numĕro 30” - give out such doses in number 30. This sentence can be shortened as follows: D. td N 30.
After the number of doses, the doctor prescribes a signature. The signature reveals the rules for using the drug. The third line of the document begins with the word Signa (indicate). It is abbreviated as follows: S. In the signature, the doctor writes how many tablets the patient should take, and how often. This concludes the writing of the prescription. After the signature there is a sign - #. It indicates that the recipe is complete.
In Latin, the prescription for Metformin is written as follows:
- Rp.: Tab. Metformini 1.0
- DTD N 30
- S. 1 tablet 2 times a day with meals
- #
First aid
Most deaths from alcohol poisoning are associated with delayed medical care. If you suspect severe intoxication, you should urgently call a doctor.
Before his arrival:
- do not leave the person alone, even if he has fallen asleep or lost consciousness. If he starts to feel sick, he may choke on the vomit;
- It is better if the patient stays awake; you should not let him fall asleep;
- if he is vomiting but cannot get up, place him on his side to prevent him from choking;
- if there are no problems with swallowing, you can give a sorbent. This will reduce the absorption of alcohol from the stomach.
What not to do:
- give a person to drink alcohol, coffee, milk, etc.;
- send him under a cold or contrast shower or into the bath;
- try to induce vomiting, rinse the stomach (danger of internal bleeding);
- force him to walk, stand on his feet (it’s better to sit him down or lay him down comfortably).
In cases of alcohol poisoning, people most often die due to breathing problems. It is associated with the accumulation of vomit in the oral cavity and trachea. If a person is not breathing, is wheezing, or wheezing, the upper respiratory tract needs to be cleared. To do this, place him on his side, and remove the vomit with his fingers without pushing it deeper. If the victim is unconscious, he should lie so that the tongue does not fall into the larynx.
Possible adverse reactions
Negative reactions can occur from various systems of the human body. In particular, disruptions in the functioning of the nervous system occur, which are characterized by impaired taste perception. Side effects may include nausea, diarrhea, and vomiting. These occurrences are accompanied by loss of appetite and pain. Sometimes taking the drug is accompanied by itching and rash on the skin. Metabolism develops much less frequently, which provokes the development of lactic acidosis. Irregularities in the liver and problems with the biliary tract were also recorded, but after stopping metformin, such manifestations disappear.
In case of overdose, an increase in blood glucose levels is not observed. But if the maximum daily dose is exceeded, the risks of an increase in lactic acid levels in the blood increase, which leads to the development of lactic acidosis. In this case, the use of the medication is stopped and the patient is hospitalized. Treatment is symptomatic. If necessary, a decision is made about hemodialysis.
Dosage and use of the product
For the treatment of type 2 diabetes mellitus in adults, it is recommended to start taking the drug with a minimum dosage. This is 500 mg 2-3 times a day. Dose adjustments are made 10-15 days after measuring glucose levels. By increasing the dose at a slow pace, it is possible to minimize the risks of disruptions in the gastrointestinal tract. A maintenance dose of metformin is considered to be 2000 mg/day, divided into 2-3 doses. It is possible to increase it to 3000 mg/day.
To improve control of blood glucose levels in patients suffering from type 2 diabetes mellitus, complex therapy with insulin is used. In this case, the initial dose is also 500 mg or 850 mg 2-3 times a day, but the dosage of insulin is selected depending on the concentration of glucose in the blood. During long-term treatment with the drug, it is necessary to perform regular glycemic control. The drug can be taken without interruption every day. If you refuse the drug, you must inform your doctor.
Taking the medication does not affect the ability to drive. This is explained by the fact that hypoglycemia does not occur when treated with it. The shelf life of the drug is 2 years. To do this, you need to choose a dark, dry place that is inaccessible to children.
Features of the impact
Metformin is a hypoglycemic agent that belongs to the biguanide group. The active substance of the same name is distinguished by its ability to reduce glucose synthesis. In addition, the drug increases sensitivity to insulin and improves the processing of glucose by cells. When taking metmorphine, it is possible to stabilize the patient’s body weight, and in some cases even reduce it.
After oral administration, the drug is slowly absorbed into the gastrointestinal tract. The maximum amount of active substance in the blood is observed after approximately a couple of hours. In this case, the bioavailability of the drug is 50-60%. If the medicine is taken with fatty foods, the absorption of the active substance slows down.
Health care
Before hospitalization the following is performed:
- breathing control. To ensure ventilation of the lungs, the oral cavity is cleared of vomit, and the tongue is fixed with a holder. It is possible to use an airway; in case of coma, intubation is necessary;
- gastric lavage. If the patient is unconscious, it is performed through a probe;
- maintenance therapy. Intravenous administration of glucose solution, vitamins, insulin or other drugs - the set is determined by the patient’s condition;
- symptomatic treatment. Relieving pain, restoring normal breathing, lowering blood pressure, etc.;
- if the body temperature is low, the person freezes - warming up (with heating pads).
After providing primary care for acute ethanol poisoning, doctors at the NarcoDoc clinic recommend hospitalization in a hospital. In the clinic, they continue to monitor the patient’s condition, treat him, and relieve possible complications of intoxication.
Contact our specialists for treatment of alcoholism followed by rehabilitation, call: +7