Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Dimethicone – has the property of reducing gas and foaming in the intestines. Thus, gas formation in the intestines is reduced and bloating is eliminated, as well as pain caused by stretching of the intestinal walls. The drug has an enveloping and absorbent effect, protecting the gastric mucosa. At the same time, complaints about discomfort caused by increased production of hydrochloric acid decrease.
Guiazulene – has pronounced antioxidant, regenerating anti-inflammatory effects.
Pepsan-R reduces the secretory activity of the gastrointestinal tract by suppressing the process of histamine release from mucosal cells.
Pharmacokinetics
No data.
Indications for use
In the symptomatic treatment of patients with complaints of pain in the stomach and esophagus caused by increased production of hydrochloric acid : pain in the stomach; disorders of the gastrointestinal tract with complaints of belching , heartburn , flatulence , nausea, stool disorders ( diarrhea / constipation ); preparing the patient for examination of the gastrointestinal tract.
Pepsan - R gel for oral administration sachet 10 g N 30
Active substance
guaiazulene
ATX code
A03AX (Other drugs used for bowel dysfunction)
Release form, packaging and composition of the drug
Capsules
blue, soft, oblong, shiny, capacity from 0.27 ml to 0.333 ml; the contents of the capsules are a viscous blue solution.
| 1 caps. | |
| dimethicone | 300 mg |
| guaiazulene | 4 mg |
[PRING] gelatin - 131.5 mg, glycerol - 31.2 mg, sorbitol 70% non-crystallizable - 21 mg, patented blue V - 0.2 mg, titanium dioxide - 1.1 mg, purified water - sufficient amount.
10 pieces. - blisters (3) - cardboard packs.
Gel for oral administration
blue in color, with a mint smell.
| 1 sachet | |
| dimethicone | 3 g |
| guaiazulene | 4 mg |
[PRING] sorbitol 70% crystallized - 1.429 g (corresponding amount of sorbitol - 1 mg), carrageenates - 0.13 g, sodium cyclamate - 0.012 g, methyl parahydroxybenzoate - 0.015 g, peppermint oil - 0.003 g, purified water - 5.407 g.
10 g - sachet (12) - cardboard packs. 10 g - sachet (14) - cardboard packs. 10 g - sachet (30) - cardboard packs.
Clinical and pharmacological group
A drug that reduces flatulence
Pharmacotherapeutic group
Carminative
pharmachologic effect
Data on the pharmacokinetics of Pepsan-R are not provided.
Indications for use
- gastralgia;
- gastroesophageal reflux disease grade 0-I;
- increased acidity of gastric juice;
- functional gastrointestinal disorders, manifested by heartburn, belching, increased gas formation, nausea, constipation and/or diarrhea or their alternation;
- preparation for x-ray, ultrasound or instrumental examination of the abdominal organs.
Dosage
The drug is taken orally.
Prescribe 1 capsule or sachet 2-3 times a day before meals or when pain occurs. The course of treatment is determined individually.
In preparation for abdominal examinations
Prescribe 1 capsule or sachet 2-3 times during the day on the eve of the study and 1 capsule or sachet in the morning on the day of the study.
Contraindications
- children under 14 years of age (capsules);
- children and adolescents up to 18 years of age (gel for oral administration);
- rare hereditary diseases, such as fructose intolerance, glucose-galactose malabsorption, sucrase-isomaltase deficiency (since the drug contains sorbitol);
- hypersensitivity to the components of the drug.
Overdose
Possible increased side effects.
Treatment:
carrying out symptomatic therapy.
Side effects
Rarely:
allergic reactions, pain, bloating.
Overdose
No drug interactions have been established.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. The shelf life of capsules is 4 years, gel for oral administration is 2 years.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Special Instructions
Pepsan-R does not contain sugar, therefore it is not contraindicated in patients with diabetes.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to perform potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions (including driving vehicles, working with moving mechanisms).
Instructions for use Pepsan-R
One sachet/capsule orally three times a day, without chewing, before meals, with 0.5 glasses of drinking water or when pain occurs. The duration of taking the drug is determined by a specialist.
When preparing a patient for gastrointestinal examinations, take Pepsan on the eve of the examination, one capsule/sachet three times a day and one capsule/sachet in the morning on the day of the hardware examination.
Instructions for use of Pepsan-R indicate the possibility of prescribing the drug during lactation and pregnancy.
Pepsan-R
Pepsan-R
(lat.
Pepsan-R
) is a combined drug that has an anti-inflammatory effect, reduces the production of acid in the stomach, and reduces the formation of gases in the intestines. The main effects of Pepsan-R (Minushkin O.N. et al.):
- decrease in histaminase and histamine levels
- suppression of mast cell degranulation
- anti-inflammatory and anti-edematous effect
- reducing the aggressive properties of refluxate (by reducing stomach acid production)
- shortening the exposure time of refluxate in the esophagus
- reducing irritation and inflammation of the esophageal mucosa by binding free radicals
- restoration of the protective layer of the esophagus and stomach mucosa
- eliminating flatulence and reducing the number of gastroesophageal refluxes
Active ingredients of Pepsan-R
Pepsan-R has two active ingredients:
- defoamer dimethicone
- a hydrophobic polymer substance with low surface tension that reduces gas formation in the intestines and covers the walls of the gastrointestinal tract with a protective film - a synthesized analogue of the main active ingredient of chamomile guaiazulene
, an anti-inflammatory, anti-allergic, antioxidant agent that stimulates regenerative processes. Guiazulene inhibits the production of histamine in the stomach, resulting in a decrease in the secretion of hydrochloric acid in the parietal cells of the gastric mucosa.
Dosage forms of Pepsan-R
In Russia, Pepsan-R is presented in two dosage forms:
- capsules, each containing 300 mg dimethicone and 4 mg guaiazulene; the capsules are soft to the touch, blue in color, shiny in appearance, oblong in shape and contain a viscous blue solution
- gel for oral administration; 1 sachet of gel contains 3 g of dimethicone and 4 mg of guaiazulene; gel color is blue, smell is mint
Indications for use of Pepsan-R
- cramping pain in the stomach (gastralgia)
- gastroesophageal reflux disease
- increased acidity of gastric juice
- functional disorders of the gastrointestinal tract: heartburn, belching, flatulence, nausea, constipation, diarrhea
- preparation for x-ray, ultrasound or instrumental examination of the abdominal organs
Procedure for using Pepsan-R and dose
Pepsan-R is taken orally, 1 capsule or 1 sachet 2-3 times a day before meals or when pain occurs.
The duration of therapy is determined by the doctor. In preparation for examinations of the abdominal organs, Pepsan-R is taken orally, 1 capsule or 1 sachet 2-3 per day preceding the examination and 1 capsule or 1 sachet in the morning on the day of the examination.
Use of Pepsan-R by pregnant women, breastfeeding and children
There are no restrictions on Pepsan-R therapy for pregnant and nursing mothers.
Pepsan-R in any form is contraindicated in children under 14 years of age. Pepsan-R in the form of an oral gel is contraindicated for children and adolescents under the age of 18 years.
general information
Pepsan-R fully complies with the requirements for modern antacids (Maev I.V. et al.): • good ability to bind hydrochloric acid in the stomach and maintain acidity at the level of 3.0-4.0 • high adsorbing capacity for bile acids , lysolecithin and pepsin • absence of the “acid rebound” phenomenon, as, for example, in antacids containing calcium or sodium carbonates • insignificant effect on mineral metabolism, gastrointestinal motility and urine acidity • minimal possible enteral absorption of aluminum and magnesium ions • minimal flatulence effect • rapid relief of pain and dyspeptic syndrome and a significant duration of action • the presence of several dosage forms, including capsules and gel form, pleasant taste.
According to the pharmacological index, Pepsan-R belongs to the groups “Carminatives” and “Other gastrointestinal drugs”. According to ATC - to the group “A02DA Carminative drugs”. There are contraindications, side effects and application features; consultation with a specialist is necessary.
The manufacturer of Pepsana-R is Laboratoires Rosa-Phytopharma, France.
Patient Materials
The GastroScan.ru website contains materials for patients on various aspects of gastroenterology:
- “Advice from doctors” in the “Patients” section of the site
- “Popular gastroenterology” in the “Literature” section
- “Popular gastroenterology” in the “Video” section
Materials for healthcare professionals regarding the use of Pepsan-R
Articles
- Maev I.V., Dicheva D.T., Lebedeva E.G. Possibilities of antacids in the treatment of chronic gastritis // Experimental and clinical gastroenterology. – 2010. – No. 10. – P. 87–92.
- Minushkin O.N., Zverkov I.V., Loranskaya I.D., Tugova Yu.E., Burdina E.G. New technology for the treatment of gastroesophageal reflux disease // Kremlin Medicine. Clinical Bulletin. 2012. No. 1. pp. 81–84.
- Dicheva D.T., Andreev D.N., Ulyankina E.V. Syndrome of overlap of GERD, functional dyspepsia and IBS: pathogenetic connections and approaches to therapy // Effective pharmacotherapy. 2021. T. 15. No. 36. P. 64-70.
- Minushkin O.N., Zverkov I.V., Lvova N.V., Skibina Yu.S., Inevatova V.S. Chronic gastritis: current state of the problem // Therapeutic archive. - 2021. - T. 92. - No. 8. - pp. 18-23.
On the website GastroScan.ru in the “Literature” section there is a subsection “Gastritis, duodenitis, erosion”, containing publications for healthcare professionals.
Video
Still from video: Shumkov Yu.P. Biliary reflux
On the website in the “Video” section there is a subsection “For Doctors”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals.
Pepsan-R has contraindications, side effects and application features; consultation with a specialist is necessary. Back to section
Analogs
Level 4 ATC code matches: Disflatil
Mint tablets
Kuplaton
Espumisan
Espumisan Baby
Espumisan L
Espumisan 40
Bobotik
Dill water
Sub Simplex
Infacol
Plantex
Bebinos
Dicetel
Meteospasmil
Romazulan
Espumisan , Meteospazmil , Simikol , Gascon , Drop , Unienzim with MPS, Pepfiz , Relzer , Softovak and others.
Pepsan-R price, where to buy
The price of Pepsan-R in capsules No. 30 ranges from 279 to 316 rubles; gel - No. 30 sachets from 343 to 395 rubles. You can purchase Pepsan-R in most pharmacies in Moscow.
- Online pharmacies in RussiaRussia
ZdravCity
- Pepsan-r caps.
n30Rosa-Phytopharm 462 RUR order
Use of the drug "Pepsan-R" in the treatment of gastroesophageal reflux disease
O. Minushkin, Doctor of Medical Sciences, Professor, I. Loranskaya, Doctor of Medical Sciences, Professor, I. Zverkov, Doctor of Medical Sciences, Professor, L. Rakitskaya, Candidate of Medical Sciences, L. Mamedova, Candidate of Medical Sciences, V. Vishnevskaya, Candidate of Medical Sciences, L. Maslovsky, Doctor of Medical Sciences,
Department of Gastroenterology, MC UD of the President of the Russian Federation,
The anti-inflammatory effect of the drug “Pepsan-R”, new to the Russian Federation, was assessed, treatment of which was received by 50 patients with gastroesophageal reflux disease (GERD) grades 0–1. Treatment was carried out in monotherapy mode. The drug contains 2 active ingredients: guaiazulene and dimethicone. Its mechanism of action is to suppress the degranulation of mast cells and create a protective layer on the mucous membrane of the esophagus and stomach. The effectiveness of the drug has been clinically demonstrated in 100% of patients (10% of them required an increase in the dose of the drug). Morphoendoscopically positive dynamics over 4 weeks were noted in 50% of patients. No side effects have been reported. Key words : gastroesophageal reflux disease, treatment, Pepsan-R.
* The drug underwent pre-registration studies at 2 centers: the Department of Gastroenterology of the MC UD of the President of the Russian Federation and the Department of Gastroenterology of the Russian Medical Academy of Postgraduate Education.
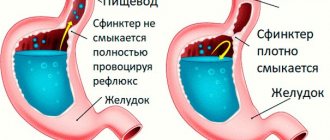
Reflux is the reflux of contents from the underlying sections of the gastrointestinal tract to the overlying ones in places of physiological narrowings (sphincters) separating physiologically and anatomically different sections of the digestive tract; reflux leads to irritation, inflammation and structural changes in the areas of reflux with the formation of certain clinical manifestations (our definition, 1986).
In clinical practice, they distinguish: gastroesophageal, duodenal-gastric reflux and reflux from the large intestine to the small intestine through the ileocecal valve.
The true incidence of reflux and its clinical significance are unknown. Maximum importance is attached to gastroesophageal reflux, which is most likely due to the most studied clinical manifestations and diagnostic approaches.
The Genval Conference (1999) stated: “The term “gastroesophageal reflux disease” can be used to encompass all individuals who are at risk of physical complications from gastroesophageal reflux or who experience clinically significant impairment of well-being (quality of life) due to reflux-related symptoms after adequate confirmation of the benign nature of these symptoms." Finally, the Montreal Congress of Gastroenterology (2006) defines gastroesophageal reflux disease (GERD) “as a condition that occurs when reflux of stomach contents causes distressing symptoms and/or complications.”
From the definitions it is clear that ideas about GERD are dynamic. This dynamism continues to this day, which is determined by unsatisfactory treatment results.
Thus, until 1995, GERD was associated with erosive reflux esophagitis, the treatment of which was a course of treatment. In 1995, it became clear that the effect of treatment was temporary, and the question arose about prolonged treatment, albeit for certain groups of patients. By 1999, the final point of view had emerged: treatment should be permanent or should begin in the early period of the disease. At the same time, the early stage of GERD was identified - non-erosive reflux disease. Did this solve all the problems? No! But new ones have been born, the solution of which is being addressed by modern gastroenterology.
It has been established that the main mechanism for the development of GERD is a dysfunction of the antireflux barrier, in which the leading role belongs to a decrease in the tone of the lower esophageal sphincter, insufficiency of the obturator mechanism of the cardia, a decrease in esophageal clearance (chemical and peristaltic) and a decrease in the resistance of the esophageal mucosa.
Increased intragastric and intra-abdominal pressure and destruction of the lower esophageal sphincter are considered factors contributing to the development of GERD; consumption of certain foods (coffee, chocolate, citrus fruits, fats) and taking medications (nitrates, antispasmodics, β-blockers, etc.). Under these conditions, reflux of gastric or duodenal contents with damaging agents takes on a decisive role. Gastric juice contains hydrochloric acid and pepsin, which, when they enter the esophageal mucosa, weaken intercellular contacts, widen intercellular spaces, disrupt intercellular interaction, which leads to damage to the esophagus. A protective reaction is the arrival of polymorphonuclear leukocytes to the site of damage, which cannot cope with the protective function, since the aggression factor is not eliminated, lipid peroxidation (LPO) products and free radicals appear, which contribute to aggression [4]. Acid aggression in the esophagus promotes the activation of fat cells, then an increase in their number and degranulation with the release of inflammatory mediators, primarily histamine [2], and, finally, a decrease in the pH of the environment triggers the mechanism of reverse diffusion of hydrogen ions. The consequence of the triggering of the damage mechanism is necrosis of the esophagus with the formation of erosions, and subsequently ulcers of the esophageal mucosa.
In some patients with GERD, it is not the acid that is released, but the alkaline content. In this case, the damaging agents are bile acids and pancreatic enzymes, which are activated in an alkaline environment, and with prolonged exposure lead to the destruction of protective mucus, damage to the epithelium, activation of inflammation, cell damage, the formation of erosive and ulcerative changes, and at later stages - to intestinal metaplasia, dysplasia, neoplasia.
Treatment of GERD primarily involves: avoiding foods that reduce the tone of the lower esophageal sphincter or have an irritating effect (fats, chocolate, mint, spices, onions, coffee, alcohol, orange and tomato juices); a recommendation to avoid a horizontal body position for 3–4 hours after eating; raising the head end of the bed; smoking cessation; loss of body weight if it is excess; refusal to wear tight clothing; if possible, stop taking medications that reduce the tone of the lower esophageal sphincter (antispasmodics, β-blockers, nitrates, anticholinergic drugs, calcium antagonists, theophylline, etc.).
Drug therapy is prescribed in case of ineffectiveness of lifestyle modification measures in the negative form of GERD and immediately in the erosive form of GERD and Barrett's esophagus. Antisecretory drugs are used as basic therapy: H2 receptor blockers or proton pump inhibitors; prokinetics are of auxiliary value. Having achieved a clinical or clinical-endoscopic effect, the patient is transferred to long-term maintenance treatment (minimum effective doses). At the same time, according to the Maastricht III recommendations, in the presence of persistent Helicobacter pylori (Hp) infection, it is necessary to carry out eradication therapy to prevent the migration of Hp, the development of infectious gastritis of the gastric body and the acceleration of mucosal atrophy.
We studied the clinical effectiveness of the drug "Pepsan-R" in the uncomplicated form of GERD (grades 0–1).
The objectives of the study included assessment of clinical and economic indicators during treatment with the drug, drug safety, and development of effective treatment regimens.
The drug "Pepsan-R" contains 2 active ingredients: guaiazulene (an azulene derivative) and dimethicone. Available in gel form or capsules for oral administration. Guiazulen is a chamomile extract. Currently, it is obtained by clinical synthesis. The mechanism of its action is represented by the suppression of degranulation of fat cells, a decrease in the level of histaminase and histamine at the tissue level, which is manifested by its anti-inflammatory effect, and at the level of smooth muscle fibers - by their relaxation. These effects are shown in the works of M. Akagi [1]. According to studies by A. Kouzounakis [3], in vitro, guaiazulene has an antioxidant effect on the membranes of liver microsomes, suppressing lipid peroxidation, which also contributes to the development of inflammation.
The second component of the drug is dimethicone, which is a hydrophobic polymer substance with low surface tension, which reduces gas formation (destroys the shell of gas bubbles and enhances gas removal), i.e. influences bloating and associated clinical manifestations.
All effects of Pepsan-R can be summarized as follows:
- reduces the level of histaminase and histamine;
- suppresses mast cell degranulation;
- has anti-inflammatory and anti-edema effects;
- reduces the aggressive properties of refluxate (reducing the acid-producing function of the stomach);
- shortens the exposure time of refluxate in the esophagus;
- reduces irritation and inflammation of the esophageal mucosa by binding free radicals;
- forms a protective layer on the mucous membrane of the esophagus and stomach;
- eliminates flatulence and reduces the number of gastroesophageal refluxes;
- acts throughout the entire “intestinal tube”.
The study included 50 patients, divided into 2 groups of 25 patients each, with an average age of 46.1±5.2 years.
Concomitant diseases were in remission, without signs of decompensation, and no additional prescriptions were required. Patients of group 1 received the drug in the form of a gel, 1 sachet after each meal; if necessary, they could take an additional sachet if the heartburn did not stop, but not earlier than 15 minutes after the 1st dose.
Patients of the 2nd group received capsules (1 after each meal; they could take an additional capsule if the effect did not occur). In 1 day, no more than 6 capsules or gel packets were allowed. The treatment lasted 28 days.
Endoscopic monitoring was carried out initially, on the 14th and 28th days of treatment. Clinical symptoms were assessed initially and over time using a 5-point Likert scale with calculation of average scores (on days 1, 14, 28 of the study):
- 1 point – no symptoms;
- 2 points – low intensity (you can’t notice it if you don’t think about it);
- 3 points – moderate severity of symptoms (cannot be ignored, but do not interfere with daytime activity or sleep);
- 4 points – severe intensity (symptoms interfere with daytime activity or sleep);
- 5 points – very strong intensity (daytime activity or sleep is impossible).
Endoscopic pH-metry was performed initially.
Statistical processing of the results included calculating the arithmetic mean and standard deviation, applying the Student's test, and determining the significance of differences between groups.
Patients kept daily diaries with an assessment of the main symptoms: heartburn, burning sensation in the chest, belching.
In the 1st group of patients who took Pepsan-R in the form of a gel, GERD was manifested by heartburn (3.5±0.17 points), belching (2.9±0.18 points), burning sensation in the sternum (2.2± 0.2 points). In group 2 (taking Pepsan-R capsules), the average Likert scale score before treatment was: for heartburn - 3.2±0.21, for belching - 2.4±0.34, for burning sensation in the chest - 1 .7±0.26. There were no significant differences between the groups in these indicators; The pH in the lower third of the esophagus in both groups was >5.
According to endoscopy, the patients were divided approximately equally (negative forms, catarrhal esophagitis; erosive esophagitis was recorded in 6 patients - single erosions).
The average dose of the drug was 3.6 capsules and 3.7 sachets per day; 6 patients received 6 sachets or 6 capsules per day (about 10% of patients).
The dynamics of clinical symptoms during treatment with Pepsan-R are presented in the table.
Assessment of clinical symptoms during treatment with Pepsan-R (M±m)
| Group | Complaints | 1st day | 14th day | Day 28 | R |
| 1st (Pepsan-R gel; n=25) | Heartburn | 3,5±0,17 | 3,0±0,21 | 2,4±0,22 | р1.14=0.0149 р14.28=0.0051 р1.28=0.00109 |
| Belching | 2,9±0,18 | 2,5±0,22 | 2,1±0,28 | р1.14=0.222 р14.28=0.1678 р1.28=0.02236 | |
| Burning behind the sternum | 2,2±0,20 | 2,0±0,37 | 1,7±0,30 | р1.14=0.4433 р14.28=0.193 р1.28=0.0149 | |
| 2nd (Pepsan-R capsules; n=25) | Heartburn | 3,2±0,21 | 2,5±0,25 | 1,6±0,27 | р1.14=0.0031 р14.28=0.00075 р1.28=0.00002 |
| Belching | 2,4±0,34 | 2,1±0,28 | 1,7±0,21 | р1.14=0.0811 р14.28=0.0367 р1.28=0.0025 | |
| Burning behind the sternum | 1,7±0,26 | 1,4±0,22 | 1,1±0,10 | р1.14=0.081 р14.28=0.0811 р1.28=0.0239 |
As can be seen from the table, on the 14th day of treatment in the 1st group of patients receiving the gel, heartburn was assessed at 3.0±0.21 points, belching – at 2.5±0.22 points, burning sensation in the sternum – at 2.0±0.37 points (differences with indicators before treatment are significant only for heartburn; p=0.0149).
In group 2 (Pepsan-R capsules), heartburn was rated at 2.5±0.25 points, belching – at 2.1±0.28 points, burning sensation behind the sternum – at 1.4±0.22 points ( the differences are significant only for heartburn before treatment and on the 14th day of treatment; p = 0.0031).
On the 28th day of treatment in group 1, the average score for heartburn was 2.4±0.22, for belching - 2.1±0.28, for burning sensation in the chest - 1.7±0.30. Only the heartburn indicator was significantly different from that on the 1st day (before treatment) and on the 14th day of treatment (p = 0.0051). For belching, a significant difference was observed only between the 1st and 28th days of treatment (p = 0.02236) and the same for burning behind the sternum (p = 0.0149).
In group 2 (capsules), after completion of treatment, the average score for heartburn was 1.6±0.27, for belching - 1.7±0.21, for burning sensation in the chest - 1.1±0.10. For heartburn, this indicator was significantly different from that on day 1 (p = 0.00075). For belching, there was a significant difference between the initial position and the 14th day of treatment (p = 0.0367), for a burning sensation in the chest - between the initial position and the 28th day of treatment (p = 0.0239).
The assessment of the therapeutic effectiveness of Pepsan-R on a Likert scale is presented in Fig. 1 and 2. The figures show that a significant decrease in the intensity of all clinical symptoms was recorded during treatment with Pepsan-R (gel, capsules).
Rice. 1. Assessment of therapeutic effectiveness on the Likert scale of the drug “Pepsan-R” (gel)
Rice. 2. Assessment of therapeutic effectiveness on the Likert scale of the drug “Pepsan-R” (capsules)
When comparing the effectiveness of treatment in groups 1 and 2 on the 28th day of treatment, a significant difference was found only for heartburn. For other indicators, no significant difference was obtained (p=0.276 and p=0.0738).
Lack of clinical effect was noted in 2 patients; In 1 patient, treatment could not be completed due to the development of urticaria (on the 25th day of treatment).
A control endoscopic study recorded positive dynamics (endoscopic signs of catarrhal esophagitis disappeared in half of the patients).
GERD today is not only the most common gastroenterological pathology, but also the most difficult in therapeutic terms. Due to the instability of the effect of a course of treatment, a permanent treatment option has been adopted as the main one, which is typical for diseases whose nature is not completely clear.
For GERD, secretion blockers are used, which reduce the aggressiveness of reflux, and agents that regulate the tone and motility of the esophagus and sphincters, reducing reflux into the esophagus. These two links are very important for the initiation and formation of the disease, but its inflammatory morphological substrate remains without direct influence.
Clinical and morpho-endoscopic data from the study allow us to hope that the drug "Pepsan-R" can take its rightful place in the complex therapy of GERD, since, thanks to its mechanism of action, it reduces the content of the inflammatory mediator - histamine, blocking the degranulation of mast cells.
The results of the study allow us to draw the following conclusions:
- Pepsan-R reduces and relieves symptoms in the vast majority of patients with GERD stage 0–I. 10% of patients need to increase the standard dose: 3 capsules (sachets) per day to 6 capsules (sachets).
- Positive endoscopic dynamics over 28 days of treatment were observed in 50% of patients.
- The drug was well tolerated; adverse reactions (urticaria) were noted in 1 (2%) patient.
- The drug can and should be used in complex therapy of GERD and in maintenance treatment.
Literature
- Akagi M., Matsui N., Mochizuki S. et al. Inhibitory 1. effect of egualen sodium: a new stable derivative of azulen on histamine release from mast cell – like cells in the stomach // Pharmacology. – 2001; 63(4):203–209.
- Barclay RL, Dinda PK, Morris GP et al. Morphological evidence of mast cell degranulation in an animal model of acid-induced esophageal mucosal injuri // Dig Dis Sci. – 1995; 40(8):1651–1658.
- Kourounakis AP, Rekka EA, Kourounakis PN Antioxidant activity of guaiazulene and protection against paracetamol hepatoxicity in rats // J. Pharm. Pharmacol. – 1997; 49(9):938–942.
- Oh TY, Lee JS, Ahn BO et al. Oxidative damages are critical in the pathogenesis of reflux esophagitis: implication of antioxodants in its treatment // Free Radic Biol Med. – 2001; 30(8):905–915.