Heptor
Hepatoprotector, has antidepressant activity. It has choleretic and cholekinetic effects. It has detoxifying, regenerating, antioxidant, antifibrosing and neuroprotective properties.
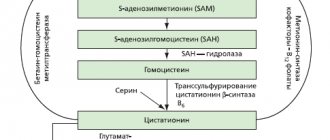
Replenishes ademetionine deficiency and stimulates its production in the body, primarily in the liver and brain. Participates in biological transmethylation reactions (methyl group donor) - the S-adenosyl-L-methionine molecule (ademetionine), is a methyl group donor in methylation reactions of phospholipids of cell membranes, proteins, hormones, neurotransmitters; participates in transsulfation reactions as a precursor of cysteine, taurine, glutathione (provides a redox mechanism for cellular detoxification), and acetylation coenzyme. Increases the content of glutamine in the liver, cysteine and taurine in plasma; reduces the content of methionine in serum, normalizing metabolic reactions in the liver. In addition to decarboxylation, it participates in the processes of aminopropylation as a precursor of polyamines - putrescine (stimulator of cell regeneration and proliferation of hepatocytes), spermidine and spermine, which are part of the structure of ribosomes.
It has a choleretic effect due to increased mobility and polarization of hepatocyte membranes due to stimulation of the synthesis of phosphatidylcholine in them. This improves the function of bile acid transport systems associated with hepatocyte membranes and promotes the passage of bile acids into the biliary system. Effective for intralobular cholestasis (impaired synthesis and flow of bile). Promotes detoxification of bile acids, increases the content of conjugated and sulfated bile acids in hepatocytes. Conjugation with taurine increases the solubility of bile acids and their removal from the hepatocyte. The process of sulfation of bile acids facilitates their elimination by the kidneys, facilitates their passage through the hepatocyte membrane and excretion in the bile. In addition, sulfated bile acids protect liver cell membranes from the toxic effects of non-sulfated bile acids (present in high concentrations in hepatocytes during intrahepatic cholestasis). In patients with diffuse liver diseases (cirrhosis, hepatitis) with intrahepatic cholestasis syndrome, it reduces the severity of skin itching and changes in biochemical parameters, incl. level of direct bilirubin, alkaline phosphatase activity, aminotransferases.
Pharmacokinetics
After a single oral dose of 400 mg, the Cmax of ademetionine in plasma is reached after 2-6 hours and is 0.7 mg/l. The bioavailability of the drug when taken orally is 5%, when administered intramuscularly - 95%.
Binding to serum proteins is negligible.
Penetrates through the BBB. Regardless of the route of administration, there is a significant increase in the concentration of ademetionine in the cerebrospinal fluid. Metabolized in the liver. T1/2 - 1.5 hours. Excreted by the kidneys.
"Heptor" in the treatment of alcoholic liver disease.
Alcohol is one of the most common causes of acute and chronic diffuse liver diseases.
An increase in alcohol consumption statistically significantly leads to an increase in mortality and a decrease in life expectancy and is one of the five most common causes of death in men in Russia and the USA. This is especially evident when analyzing mortality from liver cirrhosis, since the most vulnerable organ when exposed to alcohol is the liver. In recent years, many European countries, including Russia, have seen an increase in alcohol consumption. Russia ranks one of the first in the world in terms of the amount of alcohol consumed per year (14.6 l/year per resident of the Russian Federation), with 40-45% of the population (mostly men) drinking alcohol regularly.
Most researchers believe that drinking 40-80 g of pure ethanol per day for 10-12 years leads to a high risk of developing alcoholic liver disease (ALD). In women, the dangerous dose of ethanol is less than in men, and is 20 g per day. In addition, women are more sensitive to the toxic effects of ethanol than men; they more often develop cirrhosis of the liver against the background of alcoholic hepatitis. It has been proven that daily consumption of small doses of ethanol is more dangerous than periodic consumption of high doses, when the liver has a chance to regenerate.
The structure of ALD includes hepatic steatosis, acute and chronic alcoholic hepatitis, and cirrhosis of the liver, which can occur either alone or in combination with each other.
Alcoholic liver damage is characterized by an “erased” course of the disease; patients seek medical help when ascites, jaundice and other symptoms of the disease appear.
Judgment about the alcoholic nature of the disease is based on a number of diagnostic signs:
1. An indication of alcohol abuse or the presence of signs of chronic alcohol abuse.
2. Rapid improvement in clinical signs of the disease during abstinence.
3. Significant hepatomegaly.
4. Prevailing increase in the level of AST and γ-GT in the blood serum.
Treatment of patients with ALP should be individual and comprehensive, taking into account the severity of the condition and the potential risk of complications. Since the course of treatment for ALD is long and expensive, it is necessary to search for effective and affordable hepatoprotectors.
On the basis of the gastroenterology department of the Voronezh Regional Clinical Hospital No. 1 (chief physician - Doctor of Medical Sciences, Professor, Honored Doctor of the Russian Federation V.N. Ektov), an open clinical study was conducted on the effectiveness and safety of the drug "Heptor" (S-adenosine-L-methionine) , , Russia.
"Heptor" is a preparation of ademetionine, which is a natural substance endogenously synthesized from methionine and adenosine. Ademetionine is involved in three vital biochemical reactions: transmethylation, transsulfuration and aminopropylation, and thus plays a critical role in a complex of metabolic processes. During the formation of ALD, the first target of the action of ethanol and its toxic metabolites is the cell membranes of hepatocytes. Membranes, in addition to performing a structural function, participate in the processes of molecular transport, cell division and differentiation, and stimulate the activity of various enzyme systems. Ademetionine, being a methyl group donor, takes part in the synthesis of phosphatylcholine, the main structural component of the cell membrane. In addition, ademetionine has an antioxidant, detoxifying effect, accelerates the regeneration of liver tissue, slows down the development of fibrosis, and also has an anti-neurotoxic effect. In addition, ademetionine plays a key role in the metabolism of nucleic acids and polyamines and is a precursor of glutathione. These processes almost always suffer in liver diseases and require the administration of exogenous ademetionine. Clinical studies have shown an increase in the survival rate of patients with compensated and subcompensated alcoholic cirrhosis (Child-Pugh classes A and B) with the use of ademetionine: the two-year survival rate was 90% compared with patients receiving placebo [1]. Somewhat later, in a study by JM Mato et al. (1999) demonstrated that the use of ademetionine at a dose of 1200 mg per day in patients with compensated and subcompensated liver cirrhosis leads to a reduction in mortality and the need for liver transplantation from 29 to 12% compared with the placebo group [2]. More recent work indicates that exogenously administered ademetionine is directly involved in the processes of growth, regeneration and death of hepatocytes [3].
Thus, the choice of the drug was due to the presence of a sufficient number of clinical studies confirming the effectiveness of ademetionine in the treatment of alcoholic liver disease, on the one hand, and the affordable price of Heptor on the Russian drug market.
The study included 40 patients with Child-Pugh class A liver cirrhosis (LC), who were undergoing inpatient or outpatient treatment in the gastroenterological departments of the State Regional Clinical Hospital No. 1, in a medical facility in Voronezh.
Criteria for inclusion in the study:
- men and women aged 30 to 65 years;
- history data - consumption of dangerous doses of ethanol (>40 g of pure ethanol for men and >20 g of pure ethanol for women);
- duration of consumption of ethanol-containing drinks 8-10 years;
- patients' consent to participate in the study.
Patients were not included in the study:
- with the presence of liver cirrhosis of another etiology, the presence of liver cirrhosis of Child-Pugh classes B and C.
- the presence of severe concomitant pathology (cardiovascular, lung diseases, malignant tumors);
- incapable of abstinence during the clinical trial;
Materials and methods of research.
The study group consisted of 32 men (80%) and 8 women (20%); average age 47.3+ - 2 years, who, as a result of a comprehensive examination, were diagnosed with liver cirrhosis (class A according to the Child-Pugh diagnostic criteria).
In order to make a diagnosis and evaluate the effectiveness and safety of the drug, the following were carried out:
- questionnaire (PAS questionnaire) to identify chronic alcohol intoxication;
- assessment of changes in biochemical parameters of blood serum; activity of transaminases, alkaline phosphatase, GGTP, bilirubin, total protein and protein fractions, prothrombin, lipid spectrum indicators: cholesterol, triglycerides;
- ultrasound examination (determining the size of the liver and spleen, the diameter of the intrahepatic bile ducts, common bile duct, portal vein);
- Doppler ultrasound (determining the speed and direction of blood flow);
- esophagogastroduodenoscopy (determining the presence of varicose veins of the esophagus);
- determination of markers of viral hepatitis.
All patients in the study group were prescribed ademetionine (Heptor) orally at a dose of 800 mg/day.
The effectiveness of treatment was determined by the following parameters:
- improvement of the subjective well-being of patients;
- relief of the main clinical manifestations of liver cirrhosis;
- significant improvement in laboratory parameters: cytolysis syndrome, cholestasis syndrome;
- positive dynamics of the liver according to ultrasound.
The assessment of the safety of treatment was carried out on the basis of subjective tolerability of the Heptor drug by patients and data from laboratory research methods (CBC, FAM, blood parameters).
Clinical symptoms and laboratory data (CBC, TAM, ALT, AST, ALP, GGTP, total protein, bilirubin) were assessed at the beginning of treatment, on the 14th and 28th days of therapy; the level of albumin, PTI, cholesterol, triglycerides - at the beginning and on the 28th day of treatment with Heptor.
It should be noted that 31 patients (77.5%) showed signs of chronic pancreatitis with impaired exocrine function; in 9 (22.5%) endoscopic examination revealed erosive gastritis not associated with H. pylori. For the treatment of concomitant diseases, patients were respectively prescribed enzyme preparations without bile acids (Creon 25 thousand units per meal), proton pump inhibitors at a dose of 40 mg/day. When conducting psychometric tests, encephalopathy 0-1 was revealed in all patients, and therefore the patients were prescribed lactulose (Duphalac) in a dose of 20-30 ml (the dose was selected individually).
Results.
According to the results of the questionnaire (PAS), 28 patients (70%) gave positive answers to 18 questions and 12 patients (30%) gave positive answers to 15 questions, which suggested a high probability of systematic alcohol consumption, which contributed to the formation of ALD and cirrhosis in patients .
Figure 1. Results of analysis of PAS questionnaires (patients with alcoholic liver disease at the stage of cirrhosis, Child-Pugh class A)
The clinical picture of cirrhosis was dominated by astheno-vegetative and dyspeptic syndromes (Table 1).
Table 1
Dynamics of astheno-vegetative and dyspeptic syndromes in patients with alcoholic liver disease at the stage of Child-Pugh class A cirrhosis during treatment with the drug "Heptor"
| Sign | Number of patients with symptoms | Number of patients without symptoms | ||||
| Before treatment (n=40) | After 2 weeks (n=40) | After 4 weeks (n=40) | ||||
| n | % | n | % | N | % | |
| Weakness | 40 | 100 | 15 | 37,5 | 38 | 90 |
| Decreased performance | 40 | 100 | 18 | 45 | 34 | 85 |
| Fast fatiguability | 40 | 100 | 12 | 30 | 35 | 87,5 |
| Feeling of heaviness in the right hypochondrium | 40 | 100 | 18 | 45 | 38 | 95 |
| Belching | 40 | 100 | 17 | 72,5 | 37 | 92,5 |
| Nausea | 40 | 100 | 16 | 40 | 40 | 100 |
| Flatulence | 40 | 100 | 13 | 32,5 | 37 | 92,5 |
During the initial examination, weakness, decreased performance, fatigue, a feeling of heaviness in the right hypochondrium, belching, nausea, and flatulence were detected in all patients included in the study.
After 2 weeks from the start of treatment, the following dynamics of indicators of astheno-vegetative and dyspeptic syndromes were revealed: weakness was relieved in 15 patients (37.5%), decreased performance - in 18 (45%), fatigue - in 12 (30%) , a feeling of heaviness in the right hypochondrium - in 18 (45%), belching - in 17 (72.5%), nausea - in 16 (40%), flatulence - in 13 (32.5%). When studying patients at the end of a 4-week course of treatment, it turned out that the clinical manifestations of astheno-vegetative syndrome were relieved on average in 87.5% of patients: weakness was relieved in 36 patients (90%), decreased performance in 34 (85%), fatigue - in 35 (87.5%). Clinical manifestations of dyspeptic syndrome were relieved on average in 95% of patients: a feeling of heaviness in the right hypochondrium - in 38 (95%), belching - in 37 (92.5%), nausea - in all studied patients, flatulence - in 37 (92, 5%).
During therapy with Heptor, the manifestations of asthenovegetative and dyspeptic syndromes observed in patients with ALD at the stage of liver cirrhosis (class A according to the Child-Pugh criteria) significantly decreased.
Dynamics of laboratory data
The results of a general blood and urine test did not differ from normal values both before and after the course of treatment.
When examining patients with cirrhosis before the start of treatment, all patients revealed: an increase in the level of ALT (on average up to 3.5 N), AST (on average up to 2.2 N), an increase in bilirubin (on average up to 2.1 N) (Table 2).
table 2
Dynamics of biochemical parameters in patients during treatment with the drug "Heptor"
| Research days | |||
| Day 0 | Day 14 | Day 28 | |
| AlAt | 3.5 N | 2.9N | 1.5N |
| AsAt | 2.2 N | 1.5N | N |
| GGTP | 4.3N | 3.7N | 1.9N |
| alkaline phosphate | 2.5N | 1.8 N | N |
| Bilirubin | 2.1N | 1.7N | N (89%) |
| Albumen | 46,0±1,6 | — | 49,0±1,8 |
| PTI, % | 78,0±6,5 | — | 81,0±8,1 |
The dynamics of biochemical parameters were assessed on days 14 and 28; indicators of protein-synthetic liver function - on the 28th day of treatment.
Cytolysis rates decreased in all patients already on the 14th day of treatment and practically returned to normal by the 28th day of therapy. By the 14th day, ALT decreased on average to 2.9 N (by 17%), by the 28th day - to 1.5 N (by 53%); AST in all patients decreased compared to baseline to 1.5 N (32%) by the 14th day and by the 28th day did not exceed normal values. Positive dynamics of changes in bilirubin concentration were also noted: by the 14th day, the bilirubin level decreased to 1.7 N (by 19%), by the 28th day it was within normal limits in 89%, in 11% of patients, bilirubin was increased to 1 ,2 N. Thus, within 28 days of treatment of patients with cirrhosis, regression of the cytolytic syndrome occurred in the majority of patients.
Before the start of therapy, the patients included in the study showed an increase in the level of cholestasis markers: ALP (on average up to 2.5 N) and GGTP (on average up to 4.3 N). During treatment with Heptor, positive dynamics were noted:
- a decrease in the level of alkaline phosphatase by the 14th day of the study to 1.8 N (by 28%), by the 28th day - to normal levels;
- a decrease in the level of GGTP by the 14th day of the study to 3.7 N (by 14%), by the 28th day - to 1.9 N (by 56%);
As can be seen from the data presented in Table 2, by the 28th day of the study in the study group of patients, an increase in albumin level by 6.5% and PTI level to normal values was detected.
The initial cholesterol level in the study group of patients was on average 1.7 N, the triglyceride level was 1.5 N. As a result of the treatment, the cholesterol level decreased to normal levels by the 28th day in 83% of patients, the triglyceride level by the 28th day reached normal levels in all patients.
conclusions
In a study of the effectiveness and safety of the use of the drug Heptor in patients with alcoholic liver disease at the stage of cirrhosis (Child-Pugh class A), it was found that the use of the drug Heptor for 28 days leads to:
- reducing the severity of symptoms of asthenovegetative and dyspeptic syndromes,
- improvement of protein-synthetic function of the liver,
- reducing the severity of lipid metabolism disorders,
- positive dynamics of biochemical parameters (ALP, GGTP, AlAt, AsAt, bilirubin),
- subjective improvement in the well-being of patients.
The course of treatment revealed that the drug was well tolerated by patients.
Thus, the study showed the effectiveness and safety of the use of the drug "Heptor" in patients with alcoholic liver disease at the stage of Child-Pugh class A cirrhosis.
IN AND. Mordasova
Voronezh Regional Clinical Hospital No. 1
Literature:
1. Mato JM, Camara J, Ortiz P et al. S-adenosylmethionine in the treatment of alcoholic cirrhosis: results from a multicentric placebo-controlled, randomized double-blind clinical trial. Hepatology 1997; 26:251A.
2. Mato JM, Camara J, Fernandez de Paz J et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. Hepatology 1999; 30: 1081-1089.
3. Mato JM, Shelly C.Lu Role of S-Adenosyl-L-Methionine in liver health and injury. Hepatology 2007; 45: 1306-1312.
HEPTOR (tablets)
I somehow lost the feeling of hunger.
I didn’t feel like eating at all, neither in the morning, nor in the afternoon, nor in the evening. For some reason I always felt full. There was no “sucking” feeling in the stomach that gives you the signal “It’s time to eat.” — there is more “rash” on the face. Pimples appeared on the forehead, cheeks, and there were more blackheads. - then pain appeared under the right rib, the pain was stabbing, but weak. I immediately guessed that it was the liver that was hurting, because I had an idea where it was. Tests for “liver indicators” revealed the following - my bilirubin in the blood was increased (by the way, this was the first time I heard this word in my life) - the liver was enlarged - the outflow of bile was impaired And the verdict (i.e. diagnosis) was , as I already said “toxic hepatitis”, “Geptor” was prescribed for treatment. Before this incident, I only had to cleanse my liver a couple of times (with the drug “Ovesol”). When I came to the pharmacy and found out how much it cost, I was almost speechless. Before this, I never had to buy such expensive drugs! From the “expensive” category, half a month ago I bought myself “Melaxen” for 500 rubles, but it will last me for a whole month. I bought “Heptor” for 873 rubles!!!! And this is for some measly 20 tablets!!! And if I have to take 2 tablets 2 times a day, then this pack will last me 10 days. And you need to drink 3 packs. In short, in a month I have to cough up almost 3 thousand. Just awful. How sorry I was to give away that kind of money. But there is nothing to do, you need to get treatment.
Improvements appeared only after drinking the 1st pack of Heptor (i.e., after 10 days). When I finished the 2nd pack, I could say with confidence that my liver function had returned to normal. I didn’t know it myself before (until I felt it), but it turns out that the condition of the liver affects the entire body as a whole. Our mood, performance, sleep, and skin condition largely depend on it. After Heptor I had such a “cool” feeling. - in addition to the fact that the tests came back to normal, I became more cheerful, cheerful, my mood improved significantly, my “lazy” state went away, and the feeling of hunger finally returned. “Even my facial expression changed. Before this I looked kind of tortured. And she began to look as if she had recently been on vacation and had a good rest. -skin cleared of rashes. There is a direct relationship between the condition of your liver and the condition of the skin on your face. The cleaner the liver, the cleaner the face (and vice versa). And if it is sick or damaged, then it is very difficult for it to cleanse itself (or even stagnation of bile occurs). Despite significant improvements after only 2 packs, I still bought the 3rd and will finish this course (after all, the doctor recommended it, he probably knows better). And although at first I was sorry to pay so much money for it, the effect of the treatment is worth it!
Heptor in the treatment of alcoholic liver disease
About the article
9872
0
Regular issues of "RMZh" No. 13 dated June 15, 2010 p. 824
Category: Digestive diseases
Author: Mordasova V.I.
For quotation:
Mordasova V.I. Heptor in the treatment of alcoholic liver disease. RMJ. 2010;13:824.
Alcohol is one of the most common causes of acute and chronic diffuse liver diseases. An increase in alcohol consumption statistically significantly leads to an increase in mortality and a decrease in life expectancy and is one of the five most common causes of death in men in Russia and the USA. This is especially evident when analyzing mortality from liver cirrhosis, since the most vulnerable organ when exposed to alcohol is the liver.
In recent years, many European countries, including Russia, have seen an increase in alcohol consumption. Russia ranks one of the first in the world in terms of the amount of alcohol consumed per year (14.6 l/year per resident of the Russian Federation), with 40–45% of the population (mostly men) drinking alcohol regularly. Most researchers believe that drinking 40–80 g of pure ethanol per day for 10–12 years leads to a high risk of developing alcoholic liver disease (ALD). In women, the dangerous dose of ethanol is less than in men, and is 20 g/day. In addition, women are more sensitive to the toxic effects of ethanol than men; they more often develop cirrhosis of the liver against the background of alcoholic hepatitis. It has been proven that daily consumption of small doses of ethanol is more dangerous than periodic consumption of high doses, when the liver has a chance to regenerate. The structure of ALD includes hepatic steatosis, acute and chronic alcoholic hepatitis, and cirrhosis of the liver, which can occur either alone or in combination with each other. Alcoholic liver damage is characterized by an “erased” course of the disease; patients seek medical help when ascites, jaundice and other symptoms of the disease appear. Judgment about the alcoholic nature of the disease is based on a number of diagnostic signs: 1. An indication of alcohol abuse or the presence of signs of chronic alcohol abuse. 2. Rapid improvement in clinical signs of the disease during abstinence. 3. Significant hepatomegaly. 4. Prevailing increase in the level of AST and γ-GT in the blood serum. Treatment of patients with ALP should be individual and comprehensive, taking into account the severity of the condition and the potential risk of complications. Because Since the course of treatment for ALD is long and expensive, it is necessary to search for effective and affordable hepatoprotectors. On the basis of the gastroenterology department of the Voronezh Regional Clinical Hospital No. 1 - chief physician, MD, professor, Honored Doctor of the Russian Federation V.N. Ektov – an open clinical study of the effectiveness and safety of the drug Heptor (S-adenosine-L-methionine) was conducted, Russia. Heptor is a preparation of ademetionine, which is a natural substance endogenously synthesized from methionine and adenosine. Ademetionine is involved in three vital biochemical reactions: transmethylation, transsulfuration and aminopropylation and thus plays a critical role in a complex of metabolic processes. During the formation of ALD, the first target of the action of ethanol and its toxic metabolites is the cell membranes of hepatocytes. Membranes, in addition to performing a structural function, participate in the processes of molecular transport, cell division and differentiation, and stimulate the activity of various enzyme systems. Ademetionine, being a methyl group donor, takes part in the synthesis of phosphatylcholine, the main structural component of the cell membrane. In addition, ademetionine has an antioxidant, detoxifying effect, accelerates the regeneration of liver tissue, slows down the development of fibrosis, and also has an anti-neurotoxic effect. In addition, ademetionine plays a key role in the metabolism of nucleic acids and polyamines and is a precursor of glutathione. These processes almost always suffer in liver diseases and require the administration of exogenous ademetionine. Clinical studies have shown an increase in the survival rate of patients with compensated and subcompensated alcoholic cirrhosis (Child-Pugh classes A and B) with the use of ademetionine: the two-year survival rate was 90% compared with patients receiving placebo [1]. Somewhat later, in a study by Mato JM et al. (1999) it was demonstrated that the use of ademetionine at a dose of 1200 mg/day. in patients with compensated and subcompensated liver cirrhosis leads to a reduction in mortality and the need for liver transplantation from 29 to 12% compared to the placebo group [2]. More recent work indicates that exogenously administered ademetionine is directly involved in the processes of growth, regeneration and death of hepatocytes, on the other [3]. Thus, the choice of the drug was due to the presence of a sufficient number of clinical studies confirming the effectiveness of ademetionine in the treatment of alcoholic liver disease, on the one hand, and the affordable price of Heptor on the Russian drug market. The study included 40 patients with liver cirrhosis (LC) class “A” according to Child-Pugh, who were undergoing inpatient or outpatient treatment in the gastroenterological departments of the State Healthcare Institution VOKB No. 1, in the medical facility of Voronezh. Inclusion criteria for the study: • men and women aged 30 to 65 years; • medical history – consumption of dangerous doses of ethanol (>40 g/day of pure ethanol for men and >20 g/day for women); • duration of consumption of ethanol-containing drinks – 8–10 years; • patients' consent to participate in the study. The study did not include patients: • with liver cirrhosis of another etiology, or with Child-Pugh class B and C liver cirrhosis. • with the presence of severe concomitant pathology (cardiovascular, lung diseases, malignant tumors); • incapable of abstinence during the clinical trial. Materials and methods of the study The study group consisted of 32 (80%) men and 8 (20%) women; average age 47.3±2 years, who, as a result of a comprehensive examination, were diagnosed with liver cirrhosis (class A according to the Child–Pugh diagnostic criteria). In order to make a diagnosis and assess the effectiveness and safety of the drug, the following were carried out: • a questionnaire (PAS questionnaire) to identify chronic alcohol intoxication; • assessment of changes in biochemical parameters of blood serum; activity of transaminases, alkaline phosphatase, GGTP, bilirubin, total protein and protein fractions, prothrombin, lipid spectrum indicators: cholesterol, triglycerides; • ultrasound examination (determining the size of the liver and spleen, the diameter of the intrahepatic bile ducts, common bile duct, portal vein); • Doppler ultrasound (determining the speed and direction of blood flow); • esophagogastroduodenoscopy (determining the presence of varicose veins of the esophagus); • determination of markers of viral hepatitis. All patients in the study group were prescribed ademetionine (Heptor) orally at a dose of 800 mg/day. The effectiveness of treatment was determined by the following parameters: • improvement in the subjective well-being of patients; • relief of the main clinical manifestations of liver cirrhosis; • significant improvement in laboratory parameters: cytolysis syndrome, cholestasis syndrome; • positive dynamics of the liver according to ultrasound. The assessment of the safety of treatment was carried out on the basis of subjective tolerability of the drug Heptor by patients and data from laboratory research methods (CBC, BAM, blood parameters). Clinical symptoms and laboratory data (CBC, TAM, ALT, AST, ALP, GGTP, total protein, bilirubin) were assessed at the beginning of treatment, on the 14th and 28th days of therapy; level of albumin, PTI, cholesterol, triglycerides – at the beginning and on the 28th day of treatment with Heptor. It should be noted that 31 patients (77.5%) showed signs of chronic pancreatitis with impaired exocrine function; in 9 (22.5%) endoscopic examination revealed erosive gastritis not associated with H. pylori. For the treatment of concomitant diseases, patients were respectively prescribed enzyme preparations without bile acids (Creon 25 thousand units per meal), proton pump inhibitors at a dose of 40 mg/day. When conducting psychometric tests, encephalopathy 0–1 was detected in all patients, and therefore patients were prescribed lactulose in a dose of 20–30 ml (the dose was selected individually). Results According to the results of the questionnaire (PAS), 28 patients (70%) gave positive answers to 18 questions and 12 patients (30%) gave positive answers to 15 questions, which suggested a high probability of systematic alcohol consumption, which contributed to the formation of ALD and CPU (Fig. 1). The clinical picture of cirrhosis was dominated by astheno-vegetative and dyspeptic syndromes (Table 1). During the initial examination, weakness, decreased performance, fatigue, a feeling of heaviness in the right hypochondrium, belching, nausea, and flatulence were detected in all patients included in the study. After 2 weeks from the start of treatment, the following dynamics of indicators of astheno-vegetative and dyspeptic syndromes were revealed: weakness was relieved in 15 patients (37.5%), decreased performance - in 18 (45%), fatigue - in 12 (30%) , a feeling of heaviness in the right hypochondrium - in 18 (45%), belching - in 17 (72.5%), nausea - in 16 (40%), flatulence - in 13 (32.5%). When studying patients at the end of a 4-week course of treatment, it turned out that the clinical manifestations of astheno-vegetative syndrome were, on average, stopped in 87.5% of patients: weakness was stopped in 36 patients (90%), decreased performance - in 34 (85%), fatigue – in 35 (87.5%). Clinical manifestations of dyspeptic syndrome were relieved on average in 95% of patients: a feeling of heaviness in the right hypochondrium - in 38 (95%), belching - in 37 (92.5%), nausea - in all studied patients, flatulence - in 37 (92.5). 5%). During therapy with Heptor, the manifestations of asthenovegetative and dyspeptic syndromes observed in patients with ALD at the stage of liver cirrhosis (class A according to the Child–Pugh criteria) significantly decreased. Dynamics of laboratory data The results of a general blood and urine test did not differ from normal values both before and after the course of treatment. When examining patients with cirrhosis before the start of treatment, all patients revealed: an increase in the level of ALT (on average up to 3.5 N), AST (on average up to 2.2 N), an increase in bilirubin level (on average up to 2.1 N) (Table .2). The dynamics of biochemical parameters were assessed on the 14th and 28th days, indicators of protein-synthetic liver function - on the 28th day of treatment. Cytolysis rates decreased in all patients already on the 14th day of treatment and practically returned to normal by the 28th day of therapy. By the 14th day, ALT decreased on average to 2.9 N (by 17%), by the 28th day - to 1.5 N (by 53%); AST in all patients decreased compared to baseline to 1.5 N (32%) by the 14th day and by the 28th day did not exceed normal values. Positive dynamics of changes in bilirubin concentration were also noted: by the 14th day, the bilirubin level decreased to 1.7 N (by 19%), by the 28th day it was within normal limits in 89%, in 11% of patients, bilirubin was increased to 1 ,2 N. Thus, within 28 days of treatment of patients with cirrhosis, regression of the cytolytic syndrome occurred in the majority of patients. Before the start of therapy, the patients included in the study showed an increase in the level of cholestasis markers: ALP (on average up to 2.5 N) and GGTP (on average up to 4.3 N). During treatment with Heptor, positive dynamics were noted: • a decrease in the level of alkaline phosphatase by the 14th day of the study to 1.8 N (by 28%), achieving normal values by the 28th day; • decrease in the level of GGTP by the 14th day of the study to 3.7 N (by 14%), by the 28th day – to 1.9 N (by 56%). As can be seen from the data presented in Table 2, by the 28th day of the study in the study group of patients, an increase in albumin level by 6.5% and PTI level to normal values was detected. The initial cholesterol level in the study group of patients was on average 1.7 N, the triglyceride level was 1.5 N. As a result of the treatment, the cholesterol level decreased to normal levels by day 28 in 83% of patients, triglyceride levels reached normal by day 28 indicators for all patients. Conclusions In a study of the effectiveness and safety of the use of the drug Heptor in patients with alcoholic liver disease at the stage of cirrhosis (Child-Pugh class A), it was found that the use of the drug Heptor for 28 days leads to: • a decrease in the severity of symptoms of asthenovegetative and dyspeptic syndromes, • improvement in indicators of protein-synthetic liver function, • reduction in the severity of lipid metabolism disorders, • positive dynamics of biochemical parameters (ALP, GGTP, AlAt, AsAt, bilirubin), • subjective improvement in the well-being of patients. The course of treatment revealed that the drug was well tolerated by patients. Thus, the study showed the effectiveness and safety of the use of the drug Heptor in patients with alcoholic liver disease at the stage of Child-Pugh class A cirrhosis.
References 1. Mato JM, Camara J, Ortiz P et al. S-adenosylmethionine in the treatment of alcoholic cirrhosis: results from a multicentric placebo-controlled, randomized double-blind clinical trial // Hepatology, 1997. – Vol. 26. – P. 251A. 2. Mato JM, Camara J, Fernandez de Paz J et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial // Hepatology. – 1999. – Vol. 30. – P. 1081–1089. 3. Mato JM, Shelly C.Lu Role of S–Adenosyl–L–Methionine in liver health and injury // Hepatology. – 2007. – Vol. 45. – P. 1306–1312.
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues