Vivanat Rompharm solution for intravenous administration, 1 mg/ml syringe 3 ml 1 pc.
Registration Certificate Holder
SC ROMPHARM Company (Romania)
Dosage form
Medicine - Vivanat Rompharm
Description
Solution for intravenous administration
transparent, colorless.
1 ml
sodium ibandronate monohydrate 1.125 mg, which corresponds to the content of ibandronic acid 1 mg
Excipients
: sodium acetate trihydrate - 0.2 mg, glacial acetic acid - 0.5 mg, sodium chloride - 8.6 mg, acetic acid (1% solution) - to pH 3.7-4.0, liquid water - up to 1 ml.
3 ml - disposable syringes (1) - contour cell packaging (1) with 1 disposable needle - cardboard packs. 3 ml - disposable syringes (1) - contour cell packaging (4) with 4 disposable needles - cardboard packs.
Indications
Postmenopausal osteoporosis to prevent fractures.
Metastatic bone lesions in order to reduce the risk of hypercalcemia, pathological fractures, reduce pain, reduce the need for radiation therapy for pain and the threat of fractures.
Hypercalcemia in malignant neoplasms.
Contraindications for use
For oral and intravenous administration: hypocalcemia, severe renal dysfunction (creatinine clearance<30 ml/min), lesions of the esophagus leading to delayed emptying, such as stricture or achalasia (for oral administration), pregnancy, lactation (breast feeding), childhood and adolescence under 18 years of age, hypersensitivity to ibandronic acid.
For oral administration: lesions of the esophagus leading to a delay in its emptying, such as stricture or achalasia; inability to sit or stand for 60 minutes.
pharmachologic effect
Bone resorption inhibitor, nitrogen-containing bisphosphonate. It has a selective effect on bone tissue, which is due to its high affinity for hydroxyapatite, which makes up the mineral matrix of bone. Ibandronic acid inhibits bone resorption and has no direct effect on bone formation.
Ibandronic acid prevents bone destruction caused by gonadal suppression, retinoids, tumors and tumor extracts in vivo.
In postmenopausal women, it reduces the increased rate of bone turnover to reproductive age levels, which leads to a progressive increase in bone mass.
Ibandronic acid prevents the development of new bone metastases and reduces the growth of existing bone metastases. It has a dose-dependent inhibitory effect on tumor osteolysis.
Drug interactions
When used simultaneously with aminoglycosides, hypocalcemia may develop (since these active substances reduce serum calcium levels for a long time); hypomagnesemia is possible.
Dosage regimen
Use orally or intravenously.
The dose and regimen depend on the indications and clinical situation.
Side effect
From the digestive system:
often - dyspepsia (nausea, abdominal pain), flatulence, diarrhea, constipation, gastritis, gastroenteritis, when taken orally - esophagitis, gastroesophageal reflux disease; uncommon when taken orally - esophagitis, including esophageal ulceration or stricture, dysphagia, vomiting; rarely - duodenitis.
From the nervous system and psyche:
often - headache, dizziness, insomnia.
For the skin and subcutaneous tissues:
often - rash.
Allergic reactions:
rarely - angioedema, facial swelling, urticaria.
From the musculoskeletal system:
often - arthralgia, myalgia, pain in the limbs, osteoarthritis, back pain, musculoskeletal pain; infrequently - bone pain; rarely - atypical subtrochanteric and diaphyseal fractures of the femur (typical of the bisphosphonate class); very rarely - osteonecrosis of the jaw.
From the side of the organ of vision:
rarely - inflammatory eye diseases.
From the body as a whole:
often - flu-like syndrome, weakness; infrequently - asthenia.
Other:
often - nasopharyngitis, cystitis, urinary tract infections, bronchitis, upper respiratory tract infections, arterial hypertension, hypercholesterolemia; uncommon - reactions at the injection site, phlebitis, thrombophlebitis; rarely - hypersensitivity reactions; with intravenous administration, a short-term decrease in the level of calcium in the blood serum is possible.
special instructions
Use with caution in patients with hypersensitivity to other bisphosphonates.
During treatment, kidney function, calcium, phosphorus and magnesium levels in the blood plasma should be monitored. It is recommended to avoid overhydration in patients with circulatory failure.
It should be borne in mind that the use of bisphosphonates can cause bronchospasm in patients with bronchial asthma and with hypersensitivity to acetylsalicylic acid.
Avoid intra-arterial administration.
Products containing calcium and other polyvalent cations (for example, aluminum, magnesium, iron), incl. milk and solid foods may interfere with the absorption of ibandronic acid (they should be consumed no earlier than 30 minutes after oral administration of the drug).
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated. Restrictions when breastfeeding - Contraindicated.
Contraindicated for use during pregnancy and lactation.
Use for renal impairment
Restrictions for impaired renal function - Contraindicated.
Contraindicated in severe renal failure (serum creatinine level more than 5 mg/dL, or 442 µmol/L).
Use in children
Restrictions for children - Contraindicated.
There is no clinical experience of use in children.
Instructions for use BORIVIT® (BORIVIT)
Thiamine is completely destroyed in solutions containing sulfites.
Other vitamins are inactivated in the presence of breakdown products of B vitamins.
Pyridoxine reduces the effect of levodopa.
With parenteral use of lidocaine, in the case of additional use of norepinephrine and epinephrine, side effects on the heart may increase. In case of overdose of local anesthetic drugs, epinephrine and norepinephrine should not be used additionally.
The drug may interact with cycloserine, D-penicillamine, epinephrine, norepinephrine, and sulfonamides, which leads to a decrease in the effect of pyridoxine.
Thiamine is incompatible with oxidizing substances, mercuric chloride, iodide, carbonate, acetate, tannic acid, ferric ammonium citrate, as well as phenobarbital, riboflavin, benzylpenicillin, dextrose and metabisulfite.
Copper accelerates the breakdown of thiamine.
Thiamine loses its effect when the pH value increases (more than 3).
Vitamin B12 is incompatible with heavy metal salts.
Interactions due to lidocaine hydrochloride content
Lidocaine enhances the inhibitory effect on the respiratory center of anesthetics (hexobarbital, sodium thiopental IV), hypnotics and sedatives. When used simultaneously with hypnotics and sedatives, the inhibitory effect on the central nervous system may be enhanced. Ethanol enhances the inhibitory effect of lidocaine on respiratory function.
When used simultaneously with adrenoreceptor blockers, a reduction in the dose of lidocaine is necessary.
When used simultaneously with polymyxin B, monitoring of respiratory function is necessary.
During treatment with MAO inhibitors, lidocaine should not be used parenterally, as this increases the risk of developing arterial hypotension.
When used simultaneously with procainamide, hallucinations are possible. Lidocaine may enhance the effect of drugs that block neuromuscular transmission, since the latter reduce the conduction of nerve impulses.
In case of intoxication with cardiac glycosides, lidocaine can increase the severity of AV blockade. Lidocaine weakens the cardiotonic effect of cardiac glycosides.
Prescribe with caution with:
- β-adrenergic receptor blockers - the metabolism of lidocaine in the liver slows down, the effects of lidocaine increase (including toxic ones) and the risk of developing bradycardia and arterial hypertension increases;
- curare-like drugs - it is possible to deepen muscle relaxation (to the point of paralysis of the respiratory muscles);
- norepinephrine, mexiletine - the toxicity of lidocaine increases (the clearance of lidocaine decreases);
- isadrine, glucagon - increases the clearance of lidocaine;
- midazolam - increases the concentration of lidocaine in the blood plasma;
- anticonvulsants (ASDs), barbiturates (including phenobarbital) - it is possible to accelerate the metabolism of lidocaine in the liver, reducing the concentration in the blood;
- antiarrhythmic drugs (amiodarone, verapamil, quinidine, ajmaline, disopyramide, propafenone), PSP (hydantoin derivatives) - the cardiodepressive effect is enhanced; simultaneous use with amiodarone can lead to the development of seizures;
- novocaine, novocainamide - possible stimulation of the central nervous system and the occurrence of hallucinations;
- morphine - enhances the analgesic effect of morphine;
- prenylamine—increases the risk of developing gastric arrhythmias;
- rifampicin - a decrease in the concentration of lidocaine in the blood is possible;
- phenytoin - enhances the cardiodepressive effect of lidocaine;
- vasoconstrictors (epinephrine, methoxamine, phenylephrine) - help slow down the absorption of lidocaine and prolong its effect.
Vivanat Rompharm solution for intravenous administration 1 mg/ml syringe 3 ml N1
Action
Bone resorption inhibitor, nitrogen-containing bisphosphonate.
It has a selective effect on bone tissue, which is due to its high affinity for hydroxyapatite, which makes up the mineral matrix of bone. Ibandronic acid inhibits bone resorption and has no direct effect on bone formation. Ibandronic acid prevents bone destruction caused by gonadal suppression, retinoids, tumors and tumor extracts in vivo.
In postmenopausal women, it reduces the increased rate of bone turnover to reproductive age levels, which leads to a progressive increase in bone mass.
Ibandronic acid prevents the development of new bone metastases and reduces the growth of existing bone metastases. It has a dose-dependent inhibitory effect on tumor osteolysis.
Pharmacokinetics
After oral administration, ibandronic acid is rapidly absorbed from the upper gastrointestinal tract. The time to reach Cmax is 0.5-2 hours after administration on an empty stomach, absolute bioavailability is 0.6%. Concomitant consumption of food or drinks (except pure water) reduces the bioavailability of ibandronic acid by 90%. Consuming food or drinks 30 minutes after taking ibandronic acid reduces its bioavailability by 30%. When taking ibandronic acid 60 minutes before meals, no significant decrease in bioavailability is observed. The bioavailability of ibandronic acid is reduced to 75% when taken 2 hours after a meal.
The concentration of ibandronic acid in plasma increases in proportion to the dose of the drug taken orally (at a dose of up to 100 mg) or administered intravenously (at a dose of up to 6 mg).
After entering the systemic circulation, ibandronic acid quickly binds to bone tissue or is excreted in the urine. The apparent final Vd is 90 l, the amount of active substance in bone tissue, as a rule, reaches 40-50% of the dose circulating in the blood. Plasma protein binding at therapeutic concentrations is 87%.
There is no evidence that ibandronic acid is metabolized (in either humans or animals).
40-50% of the amount of ibandronic acid circulating in the blood penetrates the bone tissue and accumulates in it, the remaining amount is excreted unchanged by the kidneys. The magnitude of the observed apparent final T1/2 varies widely (10-60 hours) and depends on the dose and sensitivity of the analysis. The concentration of ibandronic acid in the blood decreases quickly and reaches 10% of Cmax 3 hours after intravenous administration.
The total clearance of ibandronic acid is 84-160 ml/min. Renal clearance (60 ml/min in healthy menopausal women) accounts for 50-60% of the total clearance and depends on QC. The difference between total and renal clearance reflects the uptake of the substance into bone tissue.
Vivanat Rompharm
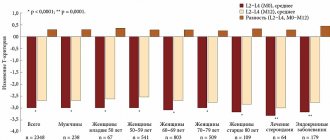
Osteoporosis can be confirmed by identifying low BMD (T index < -2 SD [Standard deviation]), fracture (including a history) or low bone mineral density (T index < - 2.5 SD) in the absence of a confirmed fracture.
Hypocalcemia
Before starting the use of Vivanat Rompharm, hypocalcemia and other disorders of bone metabolism and electrolyte balance should be corrected. Patients should consume sufficient amounts of calcium and vitamin D. If the patient does not receive enough calcium and vitamin D from food, then additional calcium and vitamin D should be taken in the form of dietary supplements.
Anaphylactic reactions
During intravenous administration of the drug, the patient's condition should be monitored, and appropriate medical care should be ensured. If an anaphylactic or other severe hypersensitivity/allergic reaction is detected, the infusion should be interrupted immediately and appropriate treatment should be initiated.
Patients with heart failure
Overhydration should be avoided in patients at risk of developing heart failure.
Patients with kidney failure
Before each injection, serum creatinine levels should be determined. Patients with underlying medical conditions receiving nephrotoxic therapy who may experience deterioration in renal function should be closely monitored.
Osteonecrosis of the jaw
When bisphosphonates were used in patients with cancer, osteonecrosis of the jaw was observed, most often associated with tooth extraction and/or local infection (in particular, osteomyelitis). Osteonecrosis of the jaw developed mainly due to the intravenous use of bisphosphonates, which was often accompanied by chemotherapy and the use of corticosteroids.
Osteonecrosis of the jaw has also been reported with oral bisphosphonates for the treatment of osteoporosis.
In the presence of associated risk factors such as cancer, radiation or chemotherapy (including angiogenesis inhibitors), taking corticosteroids, and poor oral hygiene, a dental examination and appropriate preventive treatment are recommended before prescribing bisphosphonates.
During treatment with bisphosphonates, invasive dental procedures should be avoided.
Dental surgery during bisphosphonate therapy can increase the manifestations of osteonecrosis of the jaw. It is not known whether discontinuation of bisphosphonates reduces the risk of osteonecrosis. The decision to conduct treatment should be made for each patient individually after assessing the risk/benefit ratio.
Cases of osteonecrosis of other maxillofacial areas, including the external auditory canal, have been reported in patients receiving bisphosphonate therapy, including ibandronic acid. Additional risk factors may include repeated minor injuries (eg, regular use of Q-tips). Risk factors for the development of osteonecrosis of the external auditory canal coincided with those for osteonecrosis of the jaw. Patients receiving bisphosphonates who have hearing impairment, including chronic ear infections, should be monitored for the development of osteonecrosis.
Atypical hip fractures
Atypical subtrochanteric and diaphyseal femoral fractures have been reported with bisphosphonates, primarily in patients receiving long-term treatment for osteoporosis.
Transverse and short oblique fractures can be localized along the entire length of the femur from the lesser trochanter to the supracondylar eminence. Atypical fractures occur spontaneously or as a result of minor injuries.
In the weeks or months before a complete hip fracture occurs, patients experience hip or groin pain that is often accompanied by radiographic evidence of a stress fracture.
Because atypical fractures are often bilateral, it is necessary to monitor the other hip in patients with a femoral shaft fracture.
Poor healing of atypical fractures was noted. If an atypical fracture is suspected and pending examination results, discontinuation of bisphosphonate therapy should be considered based on an assessment of the benefit/risk ratio in each individual case.
Patients should be advised to report any hip or groin pain during bisphosphonate therapy. If these symptoms are present, it is necessary to conduct an examination to identify an incomplete hip fracture.
When using bisphosphonates, incl. ibandronic acid, severe pain syndrome may occur: pain in the body and muscles. Pain occurred both within 24 hours and several months after starting the drug; in most patients it resolved after stopping therapy; in some of them, symptoms recurred after repeated use of the same or a different drug.