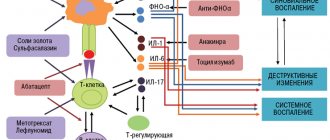
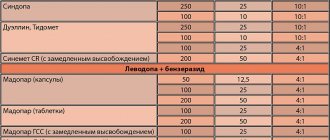
Publications in the media
(Acetazolamidum) INN
Synonyms. Diakarb, Fonurit.
Composition and release form. Acetazolamide tablets 0.25 g.
Indications. Edema resulting from pulmonary heart failure; glaucoma (various phases of development); epilepsy.
Pharmachologic effect. Acetazolamide is a carbonic anhydrase inhibitor and increases the renal excretion of bicarbonate from the body, causing an increase in urine pH. It acts in the proximal tubules of the kidneys and, by inhibiting carbonylase, leads to a decrease in the formation of carbonic acid; At the same time, the excretion of sodium and potassium from the body increases. Chloride excretion does not increase. Long-term use of acetazolamide causes a negative sodium and water balance combined with the development of hyperchloremic acidosis. Increased urinary excretion of sodium and bicarbonates significantly increases the excretion of water from the body. Acetazolamide lowers intraocular pressure, reducing the secretion of aqueous humor by 50-60% (inhibition of carbonic anhydrase of the ciliary body). The anticonvulsant effect of acetazolamide may be associated with inhibition of brain carbonic anhydrase activity and a decrease in the formation of cerebrospinal fluid.
Pharmacokinetics. The drug is quickly absorbed from the gastrointestinal tract into the blood when taken orally. The connection with plasma proteins is high - 90%. T1/2 is from 10 to 15 hours. Peak plasma concentration when taking a dose of 500 mg is reached after 2-4 hours and is 12-27 mcg/ml. The drug is excreted unchanged by the kidneys; 90 to 100% of the dose taken is excreted within 24 hours after oral administration of the tablets. The duration of action of the drug from slow-release capsules is 18-24 hours.
Side effects. Drowsiness, disorientation, paresthesia; hypokalemia, metabolic acidosis; fatigue and weakness; diarrhea; general feeling of discomfort; increased frequency of the urge to urinate; decreased appetite; metallic taste in the mouth, nausea or vomiting; weight loss; numbness, tingling or burning sensation in the hands, toes, mouth, burning sensation of the tongue, lips or anus.
Contraindications. Adrenal insufficiency (Addison's disease); hyperchloremic acidosis, hypokalemia, hyponatremia; acute liver and kidney diseases; diabetes; first trimester of pregnancy; hypersensitivity to the drug.
Adverse reactions when interacting with other drugs. When taking amphetamines or anticholinergics (especially the atropine group), or quinidine with acetazolamide, the therapeutic and side effects of the former are enhanced and prolonged as a result of a decrease in their excretion in alkaline urine. The hypoglycemic effect of oral antidiabetic agents and insulin may be reduced when taken concomitantly with acetazolamide, which can cause hyperglycemia and glycosuria in patients with diabetes mellitus. Concomitant use of ciprofloxacin and acetazolamide reduces the solubility of ciprofloxacin in the urine, and patients may experience signs of crystalluria and nephrotoxicity. The toxicity of cardiac glycosides increases when taken together with acetazolamide, which causes hypokalemia. The risk of salicylate intoxication in patients receiving large doses of salicylates increases when they are taken simultaneously with acetazolamide, since the metabolic acidosis caused by the latter increases the penetration of salicylates into the brain.
Information for the patient. Acetazolamide is recommended to be taken on an empty stomach, but if there are signs of irritation of the gastric mucosa, it can be taken with food. As a diuretic, 0.125-0.25 g is usually prescribed once a day or every other day in courses of 2-4 days. For glaucoma, 0.125-0.25 g is prescribed 1-3 times a day. Missed dose - see hydrochlorothiazide. To correct the electrolyte balance during long-term use of acetazolamide, it is necessary to administer sodium bicarbonate and potassium. In elderly patients, potassium excretion under the influence of acetazolamide increases, so it is recommended to limit yourself to 1-2 daily courses of taking the drug. Adequate fluid intake is important during acetazolamide therapy to prevent the formation of renal stones (especially in patients with hypercalciuria and gout). Patients receiving carbonic anhydrase inhibitors should include dietary supplements containing potassium or be prescribed potassium supplements.
Pharmacological properties
Pharmacodynamics.
neurotropic B vitamins have a positive effect on inflammatory and degenerative diseases of the nerves and musculoskeletal system. they are prescribed to eliminate deficiency conditions, and in high doses they have an analgesic effect, improve blood circulation, normalize the functioning of the nervous system and the process of hematopoiesis. Vitamin B1 is an important active substance. In the body, it is phosphorylated to form biologically active thiamine diphosphate (cocarboxylase) and thiamine triphosphate (TTP).
Thiamine diphosphate, as a coenzyme, takes part in important functions of carbohydrate metabolism, which are crucial in the metabolic processes of nervous tissue, and affects the conduction of nerve impulses at synapses. With a deficiency of vitamin B1, metabolites accumulate in tissues, primarily lactic and pyruvic acids, which leads to various pathological conditions and dysfunction of the nervous system.
Vitamin B6 in phosphorylated form (pyridoxal 5'-phosphate - PALP) is a coenzyme of a number of enzymes that interact in the general non-oxidative metabolism of amino acids. Through decarboxylation, they participate in the formation of physiologically active amines (for example, adrenaline, histamine, serotonin, dopamine, tyramine), through transamination - in anabolic and catabolic metabolic processes (for example, glutamate-oxaloacetate transaminase, glutamate-pyruvate transaminase, GABA, α-ketoglutarate transaminase), as well as in various processes of breakdown and synthesis of amino acids. Vitamin B6 acts on 4 different stages of tryptophan metabolism. During the synthesis of hemoglobin, vitamin B6 catalyzes the formation of α-amino-β-ketoadinic acid.
Vitamin B12 is essential for cellular metabolic processes. It affects the function of hematopoiesis (external antianemic factor), takes part in the formation of choline, methionine, creatinine, nucleic acids, and has an analgesic effect.
Pharmacokinetics. When administered orally, vitamin B6 and its derivatives are mostly rapidly absorbed in the upper digestive tract by passive diffusion and excreted within 2–5 hours. After parenteral administration, thiamine is distributed throughout the body. About 1 mg of thiamine is metabolized daily. Metabolites are excreted in the urine. Dephosphorylation occurs in the kidneys. The biological half-life of thiamine is 0.35 hours. Thiamine does not accumulate in the body due to its weak dissolution in fats.
Vitamin B6 is phosphorylated and oxidized to pyridoxal 5'-phosphate. In blood plasma, pyridoxal 5'-phosphate and pyridoxal bind to albumin. Transported in the form of pyridoxal. To pass through the cell membrane, pyridoxal 5'-phosphate bound to albumin is hydrolyzed via ALP to pyridoxal.
Vitamin B12 after parenteral administration forms transport protein complexes that are quickly absorbed by the liver, bone marrow and other organs. Vitamin B12 enters the bile and takes part in the enterohepatic circulation and penetrates the placental barrier.
Note!
Description of the drug Milgamma table. p/o No. 30 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Contraindications
Pills. hypersensitivity to the components of the drug. Taking vitamin B1 is contraindicated in case of allergic reactions. Taking vitamin B6 is contraindicated for peptic ulcers of the stomach and duodenum in the acute stage (since it is possible to increase the acidity of gastric juice).
During pregnancy and breastfeeding
Solution for injections. Hypersensitivity to the components of the drug; acute disturbance of cardiac conduction, acute form of decompensated heart failure.
Vitamin B1 is contraindicated for allergic reactions.
Vitamin B6 is contraindicated for use in cases of gastric and duodenal ulcers in the acute stage (since it is possible to increase the acidity of gastric juice).
Vitamin B12 is contraindicated for use in cases of erythremia, erythrocytosis, and thromboembolism.
Lidocaine. Hypersensitivity to lidocaine or other amide local anesthetics, a history of epileptiform seizures when taking lidocaine, severe bradycardia, severe arterial hypotension, cardiogenic shock, severe forms of chronic heart failure (II-III degree), sick sinus syndrome, WPW with-m , Adams-Stokes syndrome, AV block II and III degrees, hypovolemia, severe liver/kidney dysfunction, porphyria, myasthenia gravis.
During pregnancy and breastfeeding.
Overdose
Vitamin B1 has a wide therapeutic range. very high doses (more than 10 g) exhibit a curare-like effect, suppressing the conduction of nerve impulses.
Vitamin B6 has very low toxicity.
Excessive use of vitamin B6 in doses of more than 1 g/day for several months can lead to neurotoxic effects.
Neuropathies with ataxia and sensory disorders, cerebral spasms with EEG changes, as well as in some cases hypochromic anemia and seborrheic dermatitis have been described after administration at a dose above 2 g/day.
Vitamin B12: after parenteral administration (in rare cases, after oral administration) in doses higher than recommended, allergic reactions, eczematous skin disorders and benign acne were noted.
With long-term use in high doses, disturbances in the activity of liver enzymes, pain in the heart, and hypercoagulation are possible.
Treatment: symptomatic therapy.
Lidocaine. Symptoms: psychomotor agitation, dizziness, general weakness, decreased blood pressure, tremor, blurred vision, tonic-clonic seizures, coma, collapse, possible AV block, central nervous system depression, respiratory arrest. The first symptoms of overdose in healthy people occur at a concentration of lidocaine in the blood of 0.006 mg/kg body weight, convulsions - at 0.01 mg/kg.
Treatment: discontinuation of the drug, oxygen therapy, anticonvulsants, vasoconstrictors (norepinephrine, mesaton), for bradycardia - anticholinergics (0.5–1 mg atropine). Intubation, mechanical ventilation, and resuscitation measures are possible. Dialysis is ineffective.
Side effects
Pills
From the digestive tract: nausea, vomiting, diarrhea, abdominal pain, increased acidity of gastric juice.
From the cardiovascular system: tachycardia.
From the immune system: hypersensitivity reactions, including anaphylactic shock; anaphylaxis; hives.
Skin: skin rashes, itching.
In extremely rare cases - a state of shock.
From the nervous system: long-term use (more than 6-12 months) in doses of more than 50 mg of vitamin B6 daily can lead to peripheral sensory neuropathy, nervous agitation, dizziness, and headache.
From the endocrine system: the release of prolactin is inhibited.
Solution for injections. Long-term use (more than 6-12 months) of vitamin B6 at a dose of 50 mg daily can lead to peripheral sensory neuropathy, nervous agitation, malaise, dizziness, and headache.
From the digestive tract: gastrointestinal disorders, including nausea, vomiting, diarrhea, abdominal pain, increased acidity of gastric juice.
From the immune system: hypersensitivity reactions (skin rash, respiratory failure, anaphylactic shock, Quincke's edema), increased sweating.
Skin: itching, urticaria, acne; extremely rarely - generalized exfoliative dermatitis, angioedema.
From the cardiovascular system: tachycardia, arrhythmia, bradycardia, slowing of cardiac conduction, transverse heart block, cardiac arrest, peripheral vasodilation, collapse; very rarely - tachycardia, increase/decrease in blood pressure, heart pain.
From the nervous system: central nervous system stimulation (when used in high doses), anxiety, headache, dizziness, sleep disturbance, confusion, drowsiness, loss of consciousness, coma; in patients with hypersensitivity - euphoria, tremor, trismus, restlessness, paresthesia, convulsions.
On the part of the organ of vision: nystagmus, reversible blindness, diplopia, flickering of floaters before the eyes, photophobia, conjunctivitis.
From the organ of hearing: hearing impairment, tinnitus, hyperacusis.
From the respiratory system: shortness of breath, rhinitis, depression or respiratory arrest.
Other: sensation of heat, cold or numbness of the extremities, swelling, weakness, malignant hyperthermia, sensory disturbances, motor block.
General disorders: reactions at the injection site.
In the case of very rapid parenteral administration, systemic reactions in the form of seizures may develop.