angry at different
Human immunity is a complex multi-stage system of protecting the body from harmful external influences (viruses, bacteria, allergens, fungi). There is no single organ that is responsible for immune defense. This function is assigned to different organs of the immune system: from the lymph nodes to the intestines and ending with protein substances - immunoglobulins.
General characteristics of immunoglobulins
Immunoglobulins (Ig), or antibodies, are glycoproteins containing centers for specific non-covalent antigen binding, based on the principle of complementarity. There are soluble forms of immunoglobulins, which are called antibodies, and membrane forms of immunoglobulins, which form the basis of B-cell receptors on the surface of B-lymphocytes. Immunoglobulins are found in the blood and some secretory fluids and are produced in response to contact with antigens, such as bacteria or viruses. Sometimes immunoglobulins are produced after contact with the body's own tissues, called autoantigens.
A deficiency or excess of antibodies can be a sign of various pathologies, so determining their amount in the blood is an important part in diagnosing many diseases. In addition, modern advances in biomedicine make it possible to use synthetic antibodies in the treatment of certain diseases.
Circulating immune complexes (CIC)
CECs are circulating immune complexes, the level of which increases during acute infections and autoimmune diseases. Circulating immune complexes (CICs) are present in many people with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), especially those with complications such as vasculitis. There is a positive correlation (* systematic and conditional relationship) between disease activity and the level of CEC in the blood. CEC formation is a physiological defense mechanism resulting in the rapid elimination of either endogenous or exogenous antigens (eg, microorganisms, viruses, parasites, plant antigens, fungal antigens, pollen or food antigens) through the reticuloendothelial system. High levels of CEC in serum and/or other biological fluids are observed in many inflammatory and malignant diseases, which can cause the development of pathology. Determination of serum CEC is an important marker for assessing disease activity, especially in autoimmune diseases.
Antibody structure
Content:
- General characteristics of immunoglobulins
- Antibody structure
- Types of immunoglobulins
- Characteristics of different classes of immunoglobulins
- The role of immunoglobulins in the body
- Antibodies and immunological memory
- How to determine the amount of antibodies
- Immunoglobulins and vaccination
- Antibodies in medicine
Immunoglobulins are symmetrical Y-shaped molecules consisting of two long heavy ones (H) and two short light ones (L). chains that are connected to each other either by disulfide (SS) or hydrogen bonds. Each immunoglobulin molecule contains at least two identical H chains. The heavy chains of immunoglobulins of different classes consist of four or five domains and are designated by the letters of the Greek alphabet according to the Latin abbreviation of the class. The belonging of an antibody to a specific class and subclass is called an isotype, which is designated by the type of heavy chain. Light chains are built from two domains. They necessarily contain two types of domains - variable (V - variable) and constant (C-copstapt). Immunoglobulins produced by different clones of plasma cells have variable domains of different amino acid sequences. The constant domains are similar or very similar for each immunoglobulin isotype. Variable domains are N-terminal. As part of the light chain, the N-terminal domain is variable (VL), and the C-terminal domain is constant (CL). Heavy chains have one variable (N-terminal) domain (Vn) and several constant domains. The light and heavy chains of immunoglobulins are glycosylated.
In other words, each antibody approaches the antigen like a key and lock, and when combined, they form immune complexes. But antibodies are also capable of exhibiting the flexibility of foreign agents, making it easy to adapt to various antigens. However, this ability of immunoglobulins sometimes provokes cross-allergic reactions in a person - when the immune system of a person with allergies cannot distinguish between allergens. For example, a person with a pollen allergy due to an immunoglobulin “error” may also react to raw fruits and vegetables.
Bibliography
- Arnold JN, Wormald MR, Sim RB, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins // Annu Rev Immunol 2007; 25:21.
- Balzar S, Strand M, Rhodes D, Wenzel SE. IgE expression pattern in lung: relation to systemic IgE and asthma phenotypes // J Allergy Clin Immunol 2007; 119:855.
- Bellou A, Kanny G, Fremont S, Moneret-Vautrin DA. Transfer of atopy following bone marrow transplantation // Ann Allergy Asthma Immunol 1997; 78:513.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter // Ann Allergy Asthma Immunol 2008; 100:S1.
- Cameron L, Gounni AS, Frenkiel S, et al. S epsilon S mu and S epsilon S gamma switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination // J Immunol 2003; 171:3816.
- Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients // J Immunol 2003; 171:5602.
- Cooper AM, Hobson PS, Jutton MR, et al. Soluble CD23 controls IgE synthesis and homeostasis in human B cells // J Immunol 2012; 188:3199.
- Dullaers M, De Bruyne R, Ramadani F, et al. The who, where, and when of IgE in allergic airway disease // J Allergy Clin Immunol 2012; 129:635.
- Eckl-Dorna J, Pree I, Reisinger J, et al. The majority of allergen-specific IgE in the blood of allergic patients does not originate from blood-derived B cells or plasma cells // Clin Exp Allergy 2012; 42:1347.
- Egger C, Horak F, Vrtala S, et al. Nasal application of rBet v 1 or non-IgE-reactive T-cell epitope-containing rBet v 1 fragments has different effects on systemic allergen-specific antibody responses // J Allergy Clin Immunol 2010; 126:1312.
- Fregonese L, Patel A, van Schadewijk A, et al. Expression of the high-affinity IgE receptor (FcepsilonRI) is increased in fatal asthma // Am J Respir Crit Care 2004; 169:A297.
- Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination // Nat Rev Immunol 2003; 3:721.
- Gergen PJ, Arbes SJ Jr, Calatroni A, et al. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006 // J Allergy Clin Immunol 2009; 124:447.
- Granada M, Wilk JB, Tuzova M, et al. A genome-wide association study of plasma total IgE concentrations in the Framingham Heart Study // J Allergy Clin Immunol 2012; 129:840.
- Hamilton RG, Franklin Adkinson N Jr. In vitro assays for the diagnosis of IgE-mediated disorders // J Allergy Clin Immunol 2004; 114:213.
- Hamilton RG, Williams PB, Specific IgE Testing Task Force of the American Academy of Allergy, Asthma & Immunology, American College of Allergy, Asthma and Immunology. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist // J Allergy Clin Immunol 2010; 126:33.
- Hamilton RG. Accuracy of US Food and Drug Administration-cleared IgE antibody assays in the presence of anti-IgE (omalizumab) // J Allergy Clin Immunol 2006; 117:759.
- Hibbert RG, Teriete P, Grundy GJ, et al. The structure of human CD23 and its interactions with IgE and CD21 // J Exp Med 2005; 202:751.
- Holgate S, Casale T, Wenzel S, et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation // J Allergy Clin Immunol 2005; 115:459.
- Kamemura N, Tada H, Shimojo N, et al. Intrauterine sensitization of allergen-specific IgE analyzed by a highly sensitive new allergen microarray // J Allergy Clin Immunol 2012; 130:113.
- Lynch NR, Hagel IA, Palenque ME, et al. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment // J Allergy Clin Immunol 1998; 101:217.
- Martins TB, Bandhauer ME, Bunker AM, et al. New childhood and adult reference intervals for total IgE // J Allergy Clin Immunol 2014; 133:589.
- McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children // Infect Immun 1999; 67:484.
- Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma // N Engl J Med 2010; 363:1211.
- Niederberger V, Ring J, Rakoski J, et al. Antigens drive memory IgE responses in human allergy via the nasal mucosa // Int Arch Allergy Immunol 2007; 142:133.
- Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis // J Allergy Clin Immunol 2001; 107:429.
- Pien GC, Orange JS. Evaluation and clinical interpretation of hypergammaglobulinemia E: differentiating atopy from immunodeficiency // Ann Allergy Asthma Immunol 2008; 100:392.
- Poole JA, Meng J, Reff M, et al. Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects // J Allergy Clin Immunol 2005; 116:780.
- Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins // J Allergy Clin Immunol 2010; 125:S41.
- Selb R, Eckl-Dorna J, Twaroch TE, et al. Critical and direct involvement of the CD23 stalk region in IgE binding // J Allergy Clin Immunol 2017; 139:281.
- Shade KT, Platzer B, Washburn N, et al. A single glycan on IgE is indispensable for initiation of anaphylaxis // J Exp Med 2015; 212:457.
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils // J Allergy Clin Immunol 2010; 125:S73.
- Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma // J Allergy Clin Immunol 2007; 119:213.
- Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis // J Immunol 2005; 174:5024.
- Thorpe SJ, Heath A, Fox B, et al. The 3rd International Standard for serum IgE: international collaborative study to evaluate a candidate preparation // Clin Chem Lab Med 2014; 52:1283.
- Tsicopoulos A, Joseph M. The role of CD23 in allergic disease // Clin Exp Allergy 2000; 30:602.
- Vercelli D. Genetic regulation of IgE responses: Achilles and the tortoise // J Allergy Clin Immunol 2005; 116:60.
- Walker SA, Riches PG, Wild G, et al. Total and allergen-specific IgE in relation to allergic response pattern following bone marrow transplantation // Clin Exp Immunol 1986; 66:633.
- Watanabe N, Katakura K, Kobayashi A, et al. Protective immunity and eosinophilia in IgE-deficient SJA/9 mice infected with Nippostrongylus brasiliensis and Trichinella spiralis // Proc Natl Acad Sci USA 1988; 85:4460.
- Weidinger S, Gieger C, Rodriguez E, et al. Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus // PLoS Genet 2008; 4:e1000166.
Types of immunoglobulins
In the human body, immunoglobulins are presented in two forms:
- soluble (produced by plasma cells);
- associated with the outer membrane of B-lymphocytes, they are also receptor antibodies.
In addition, there are different classes and subclasses (isotopes) of immunoglobulins. They differ in their biological characteristics, structure and targeting. Based on differences in heavy chain structure, several classes of antibodies have been identified. Each of them has different functions and responses.
In placental mammals, including humans, 5 main classes of antibodies have been identified: IgA, IgD, IgE, IgG and IgM. Human blood contains only three of them - IgA, IgG and IgM. But the rest, according to experts, are no less useful for maintaining the immune system. They all differ in the type of heavy chain. For example, IgG molecules have gamma chains, IgM have mu chains, IgA have alpha chains, IgE have epsilon chains, and IgD have delta chains. These differences allow immunoglobulins to participate in different types and stages of immune responses.
In addition to the main classes of immunoglobulins, there are several subclasses. The difference between them is based on minor differences in the type of heavy chains in each class. There are 4 subclasses of antibodies found in the human body. The numbering corresponds to the order of decreasing their concentration in the serum. Thus, IgG and IgA antibodies are further grouped into subclasses IgG1, IgG2, IgG3, IgG4, as well as IgA1 and IgA2.
Most antibodies (IgG, IgD, IgE) in the body are presented in the form of a monomer (one molecule). The exception is class A antibodies, which also occur in the form of a dimer, and IgM, which forms a snowflake shape (pentamer).
IgE level measurement
Total IgE.
Levels of total immunoglobulin E (IgE) are often measured using a sandwich assay. In this method, an anti-IgE antibody binds to a solid support. The patient's serum is added and then the unbound protein is washed away. A second labeled anti-IgE antibody is added and the amount bound to the patient's IgE is measured. Total serum IgE levels are expressed in international units or nanograms per milliliter (1 IU/mL = 2.44 ng/mL). IgE tests are calibrated against the World Health Organization's third International IgE Reference Preparation (WHO IgE-IRP).
Allergen-specific IgE.
The first commercial test for allergen-specific IgE was the radioallergosorbent test (RAST). Bound allergen-specific IgE was detected using radioiodine and quantified using a gamma counter. The term "RAST" is still often used to refer to in vitro tests for allergen-specific IgE, although modern methods use enzymes instead of radionucleotides. A number of other technical advances in assay technology have dramatically increased the sensitivity and specificity of allergen-specific IgE measurements. The terms “in vitro IgE antibody assay” or “allergen-specific IgE immunoassay” more accurately characterize the test options that are most common in the world:
- HYTEC-288 is a colorimetric assay using a solid phase support on a paper disk. It is increasingly being replaced by the Falcon laboratory automaton.
- ImmunoCAP is a fluorinated immunoassay with a solid-phase cellulose sponge matrix.
- The Immulite chemiluminescent assay contains a biotinylated allergen and a solid phase of avidin particles.
The lower limit of detection of allergen-specific IgE is lower than that of total IgE. Most laboratories use a lower limit of approximately 0.1 to 0.35 IU/mL.
Characteristics of different classes of immunoglobulins
IgA class
About 15% of the antibodies contained in the body of a healthy person are immunoglobulins of the IgA class, which are divided into two subclasses of IgA - IgA1 and IgA2. They differ in the molecular weight of the heavy chains and the concentration in serum, where IgA is present mainly as a monomer, with a molecular weight of 160 kDa. In secretory fluids, immunoglobulins are present in the form of a dimer formed by two monomers, their content is 10-15% of the total amount of serum immunoglobulins. Dimeric immunoglobulins are present in most secretory fluids, including the mucous membranes of the respiratory and genitourinary tracts, gastrointestinal tract, as well as saliva, tears, colostrum and milk in women. Since IgA is present on the mucous membranes of the digestive system, where it can be exposed to enzymes, it contains a special component that protects the molecule from premature destruction.
Class A immunoglobulins, as a rule, are not specific in terms of “adjusting” to a specific antigen. Typically, antibodies from this group are present in vulnerable areas of the body or in areas where germs can easily enter. Class A immunoglobulins provide local humoral immunity. This is due to their properties to prevent the penetration of pathogens through epithelial surfaces; due to the presence of IgA in the secretions of mucous membranes (saliva, tears), it protects the body from some local infections.
The main function of immunoglobulins of this class is not to destroy antigens, but to prevent the penetration of infectious agents into the circulatory system. By themselves, IgA is not capable of destroying bacteria on its own, so it always works together with lysozymes, enzymes that are also present in secretory fluids and can destroy bacteria.
Disturbances in the concentration of immunoglobulins of the IgA class in the body contribute to its susceptibility to infectious diseases of the respiratory tract and genitourinary system, including nephropathy. Individuals with IgA deficiency are more prone to autoimmune disorders such as rheumatoid arthritis, lupus, allergies and asthma.
Various diseases can lead to a decrease in IgA levels, including gonorrhea. The bacteria that cause gonorrhea produce an enzyme that breaks down IgA into two parts: Fc and Fab fragments. Interestingly, Fab can still find bacteria that are dangerous to the body, but without interacting with Fc, it is not able to resist them.
IgD class
Class D immunoglobulins are present in very small quantities in the human body and make up approximately 0.2% of all antibodies. IgD is known to attach to the surface of some B lymphocytes as a B cell receptor. However, its functions in the human body have not yet been fully studied. It is assumed that IgD is the cause of allergy to penicillin, and it may also be involved in triggering autoimmune reactions.
IgE class
IgE immunoglobulin normally makes up no more than 0.1% of the total amount of serum immunoglobulins. More than 90% of IgE synthesized by plasma cells is secreted in the mucous exocretes of the gastrointestinal tract. The biological function is to protect against extracellular parasites, although this is not fully understood, and a sharp increase in the amount of IgE is a pathogenetic feature in allergic reactions.
Immunoglobulins of this group bind to the surface of basophils and mast cells. Then the antigen attaches to them, which in turn leads to the release of vasoactive amines into the bloodstream and the development of an IgE-dependent allergic reaction according to the following mechanism.
Various antigens such as pollen, toxic substances, fungal spores, dust mites or pet dander bind to IgE and trigger the release of heparin, histamine, proteolytic enzymes, leukotrienes and cytokines. This leads to the dilation of blood vessels and an increase in their permeability, which facilitates the penetration of foreign agents into the capillaries, and then into nearby tissues, as a result of which symptoms characteristic of an allergic reaction develop. However, most typical allergic reactions in the form of sneezing, coughing, watery eyes and increased mucus production help remove remaining allergens from the body.
Studies have shown that disorders such as asthma, rhinitis, eczema, urticaria and dermatitis cause increased IgE levels. E-type antibodies are also actively produced in response to the presence in the body of helminths, persistent infections (herpes viruses, atypical microorganisms) and some arthropods (for example, lice). In addition, IgE plays an indirect role in the immune response by stimulating other immune components. It can also protect the surfaces of mucous membranes, causing inflammatory reactions in case of danger.
Pathologically low levels of IgE antibodies can occur against the background of a rare genetic disease accompanied by impaired muscle coordination (ataxia telangiectasia).
IgG class
Class G immunoglobulins are dominant in the human body. They account for 75% of all antibodies. This is partly due to the long half-life: from 7 to 23 days (depending on the subclass). In addition, they can persist in the blood for several decades after contact with the antigen.
There are 4 subclasses of IgG:
- IgG1 makes up 60 to 65% of the total immunoglobulin of this class. Its deficiency is usually a sign of hypogammaglobulinemia (plasma cell deficiency).
- IgG2 is the second most common isotope, accounting for 20-25% of the total IgG. “Adult” antibody concentrations usually appear by 6-7 years of age. IgG2 deficiency has been associated with recurrent respiratory tract infections.
- IgG3 accounts for 5 to 10% of total IgG. Plays a major role in immune reactions against protein or polypeptide antigens.
- IgG4 accounts for up to 4% of the total share of IgG. Previously, IgG4 was associated only with food allergies, but recent studies have shown that increased serum IgG4 is observed in patients suffering from sclerosing pancreatitis, cholangitis and interstitial pneumonia. However, the exact role of IgG4 is still unknown.
IgG plays a key role in the humoral immune response. This is the main immunoglobulin found in the blood, as well as in the lymphatic, cerebrospinal and abdominal fluids. Its ability to remain in the body for a long time makes it the most useful antibody for passive immunization. This is the only antibody that can cross the mother's placenta and enter the fetal circulation, providing postpartum protection to the newborn during the first months of his life.
Main functions of IgG:
- increased phagocytosis in macrophages and neutrophils;
- neutralization of toxins;
- virus inactivation;
- destruction of bacteria.
IgM class
IgM is the most important member of the human immunoglobulin family, although it has a very short half-life of about 5 days.
Immunoglobulins of the IgM class in the total proportion of serum antibodies in the human body are approximately 10-13%. They participate in primary immune reactions and have pronounced antibacterial activity, the ability to fix complement, and do not penetrate the placental barrier. They are the first to be synthesized in response to antigenic stimulation of the body. The earliest antibodies belong to class M immunoglobulins, which are often used in the diagnosis of infectious diseases. They are the first to appear in the process of ontogenesis and phylogenesis.
An increase in IgM levels can be regarded as a sign of a recent infection or the presence of an antigen in the body. On the membrane of B-lymphocytes there is a monomeric form of IgM, which functions as the main component of the B-cell receptor.
Content
- What is immunoglobulin E
- Immunoglobulin E and allergies
- IgE level measurement
- Change in IgE levels
- Different levels of IgE in allergies
- Allergen-specific and general IgE
- Bibliography
The pathogenesis of many allergic diseases involves an “allergic” antibody or immunoglobulin E (IgE). It plays an important role in protection against parasitic diseases, especially those caused by helminths and some protozoa. IgE is not believed to play an important role in protection against bacterial infections, but is actively involved in the pathogenesis of allergic diseases, especially in the activation of mast cells and basophils, as well as in the presentation of antigens.
The role of immunoglobulins in the body
Antibodies are part of the humoral immune response and act very specifically, as they are always directed against a specific antigen.
The task of any antibody in the human body is to participate in immune reactions. Immunoglobulins have the ability to form immune complexes with antigen molecules, activate the complement system (a complex of proteins contained in the blood necessary to protect the body from foreign agents) and cause inflammation. All these actions are aimed at neutralizing the antigen and safely removing it from the body.
Due to different structural properties, different classes of antibodies can perform specialized functions:
- neutralize parasites (IgE);
- neutralize microorganisms (IgM, IgG);
- protect against recurrent diseases such as mumps (IgG);
- protect mucous membranes (IgA);
- participate in the synthesis of lymphocytes (IgD);
- protect the fetus (IgG) and newborn baby (IgA).
Allergen-specific and general IgE
The ratio of allergen-specific immunoglobulin E (IgE) to total IgE has been called “specific activity.” Its utility has been studied in diagnosing several allergic disorders and predicting individual response to monoclonal antibody therapy (omalizumab). One study found that specific activity is no more useful than allergen-specific IgE alone in diagnosing food allergies. However, in the treatment of asthma with omalizumab, specific activity exceeding 3-4% predicted a decrease in response to omalizumab therapy. The ratio of allergen-specific IgE to total IgE remains primarily a clinical research tool; its overall practical utility has not yet been established.
Antibodies and immunological memory
The immune response is divided into primary and secondary. The primary response occurs during the first contact with the antigen, after which the body first produces IgM immunoglobulins, which are then replaced by more specific and stable IgG antibodies.
A secondary immune response occurs upon repeated exposure to the same antigen. It is more intense than the primary one, the concentration of antibodies reaches higher levels than the first time.
This effect is due to immunological memory, which is mediated by B lymphocytes. These are long-lived cells that come into contact with the antigen, begin to divide very intensively and produce specific antibodies.
Different levels of IgE in allergies
A total serum IgE level of 100 international units/mL (IU/mL) is generally considered the upper limit of normal for older adolescents and adults. Elevated levels of total serum IgE are observed in several types of diseases, including allergic diseases, some primary immunodeficiencies, parasitic and viral infections, some inflammatory diseases, some malignancies and a number of other diseases. Thus, elevated total serum IgE is not specific for allergic disease.
Increased level of total serum IgE.
Many patients with allergic disorders have elevated total IgE levels, but there is no defined cutoff value that distinguishes patients with allergic disease from those without. Total IgE alone is rarely sufficient to diagnose an allergic disease. The following studies are illustrative:
- Adults with IgE >66 IU/mL have a 37-fold greater risk of developing allergen-specific IgE antibodies to aeroallergens compared with patients with the lowest total IgE levels.
- In one study, school-age children had a mean IgE level of 51 IU/mL. In children with atopic dermatitis and asthma, the average IgE level was 985 IU/ml; those with asthma only, 305 IU/ml; patients with eczema only - 273 IU/ml, and patients with allergic rhinitis - 171 IU/ml.
- Among atopic patients of all ages, IgE levels are highest in patients with atopic dermatitis, followed by atopic asthma, chronic allergic rhinitis, and seasonal allergic rhinitis.
- Using a total serum IgE level of 100 IU/mL, the sensitivity was 78% for patients with asthma and 60% for rhinitis to distinguish allergic from nonallergic patients. 20% of patients were incorrectly classified.
Decreased or normal levels of total IgE.
Low levels of total serum IgE cannot be used to rule out the presence of atopic disease due to the significant overlap in total serum IgE between atopic and nonatopic populations. Patients with low or normal serum IgE levels may still have local tissue production of allergen-specific IgE.
How is the amount of antibodies determined?
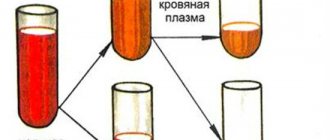
Antibodies make up 12% to 18% of whey proteins. The quantities of individual protein fractions in laboratory conditions are determined on the basis of proteinograms.
An antibody test using enzyme-linked immunosorbent assay (ELISA), as a rule, is carried out with venous blood (allows you to determine the amount of immunoglobulins of the IgM, IgG, IgE, IgA classes). In addition, the amount of IgA class antibodies can be determined by biochemical examination of a person’s saliva or feces using the polymerase chain reaction (PCR) method. In some situations, the test may be performed using other material, such as cerebrospinal fluid.
If a critical increase in certain immunoglobulins is diagnosed in the patient’s blood, they speak of hypergammaglobulinemia. As a rule, in such patients, IgM antibodies are excessively increased, while the rest remain deficient.
Against the background of a pathological increase in certain antibodies, various diseases can develop, including:
- acute and chronic inflammation;
- parasitic, bacterial, viral or fungal diseases;
- autoimmune diseases;
- cirrhosis of the liver;
- sarcoidosis;
- AIDS.
A pathologically low amount of antibodies in the serum may occur against the background of:
- congenital genetic disorders;
- taking certain antimalarial, cytostatic, glucocorticoid drugs;
- malnutrition;
- infections, including HIV;
- oncological diseases;
- nephrotic syndrome;
- extensive burns;
- severe diarrhea.
What else is prescribed with this study?
Humoral immunity (immunoglobulins IgA, IgM, IgG, IgE, circulating immunocomplexes, complement components C3, C4)
17.51. Ven. blood 8 days
3,890 ₽ Add to cart
Immune status (screening) (Phagocytic activity of leukocytes, cellular immunity, total immunoglobulin IgE, immunoglobulins IgA, IgM, IgG)
27.960. Ven. blood 3 days
7,640 ₽ Add to cart
Total immunoglobulin IgE
17.2. Ven. blood 1 day
670 ₽ Add to cart
Cellular immunity (T-lymphocytes, T-helpers, T-cytotoxic cells, Immunoregulatory index, B-lymphocytes, NK-T cells, NK cells, Leukocyte formula)
17.50. Ven. blood 3 days
5 200 ₽ Add to cart
Serum paraprotein typing using immunofixation
50.28.2181 Ven. blood 14 days
3,720 ₽ Add to cart
Immunoglobulins and vaccination
Best materials of the month
- Coronaviruses: SARS-CoV-2 (COVID-19)
- Antibiotics for the prevention and treatment of COVID-19: how effective are they?
- The most common "office" diseases
- Does vodka kill coronavirus?
- How to stay alive on our roads?
Antibodies play a key role in the development of immunity after vaccination. As a result of contact with the antigen contained in the vaccine, the immune system produces antibodies. First, less persistent and specific IgM, and then more persistent IgG. For example, during vaccination against the hepatitis B virus, the vaccine is administered three times with a certain interval between vaccinations. This allows you to create lasting immunity to the disease. The effectiveness of such vaccination is determined by changes in the amount of IgG antibodies in the body.
How to lower immunoglobulin E levels
There are no special medications to reduce the level of immunoglobulin E. Immunologists at the Yusupov Hospital draw up an individual treatment regimen for each patient suffering from allergic diseases. After a course of therapy, the level of immunoglobulin decreases.
In case of food allergies, products that cause allergic reactions are excluded from the patient’s diet, anti-inflammatory and antimediator therapy (antihistamines), enzymatic medications are prescribed, and secondary intestinal dysbiosis is corrected. Patients suffering from lupus erythematosus are prescribed painkillers, immunosuppressants, anti-inflammatory drugs and corticosteroids. For atopic bronchial asthma, strictly individual and justified complex treatment is prescribed:
- hypoallergenic diet and treatment and prophylactic regimen;
- external and systemic drug therapy;
- physiotherapeutic procedures;
- psychotherapy.
Patients with atopic bronchial asthma at the Yusupov Hospital are treated by a pulmonologist and an allergist-immunologist. Drug therapy for atopic asthma includes desensitizing and anti-inflammatory drugs. To relieve acute attacks, asthma doctors use bronchodilators. For bronchial asthma, preference is given to inhaled forms of steroids, which are used in the form of metered-dose aerosol inhalers or nebulizer therapy. To improve bronchial patency, expectorants are prescribed.
For atopic bronchial asthma, doctors at the Yusupov Hospital use plasmapheresis; outside of exacerbations, they carry out specific hyposensitization, immunocorrection, physical therapy, acupuncture and physiotherapeutic procedures.
Make an appointment with an immunologist-allergist by calling the Yusupov Hospital. You will be tested for immunoglobulin E. After a comprehensive examination, the doctor will prescribe individual treatment, after which the level of immunoglobulin E will decrease.