Key Facts
- Hepatitis D virus is a virus that requires the presence of hepatitis B virus to replicate. Hepatitis D virus infection occurs either simultaneously with hepatitis B virus infection (coinfection) or after hepatitis B virus infection (superinfection).
- Globally, nearly 5% of all people with chronic hepatitis B are infected with the hepatitis D virus; The hepatitis D virus is transmitted through the blood or other body fluids of infected people.
- There are several pockets of high prevalence of hepatitis D, including in Mongolia, the Republic of Moldova and several countries in West and Central Africa.
- Groups at high risk of co-infection with hepatitis B and D viruses include indigenous peoples, hemodialysis patients and people who inject drugs.
- Since the 1980s, there has been a decline in the overall incidence of hepatitis D worldwide. This trend is mainly due to the success of the global hepatitis B vaccination program.
- Coinfection with hepatitis D and hepatitis B viruses is considered the most severe form of chronic viral hepatitis due to the more rapid progression of fatal liver failure or hepatocellular carcinoma.
- To date, treatment success rates for this form of infection are generally low.
- Hepatitis D infection can be prevented through hepatitis B immunization.
Hepatitis D is an inflammatory liver disease caused by the hepatitis D virus, which requires the presence of the hepatitis B virus to replicate. Without hepatitis B, infection with hepatitis D is impossible.
Co-infection with hepatitis D and B viruses is considered the most severe form of chronic viral hepatitis due to the more rapid progression of fatal liver failure or hepatocellular carcinoma. The only way to prevent hepatitis D is vaccination against hepatitis B.
Viral hepatitis - symptoms and treatment
It is advisable to consider the detailed pathogenesis of each type of viral hepatitis in separate articles. It is enough to understand the options for normal functioning of the liver and the general mechanisms of its pathology.
The formation and metabolism of bilirubin is normal
Bilirubin is a product of the transformation of hemoglobin (Hb), a kind of “waste” that must be removed from the body. It is formed mainly from Hb, which is released from red blood cells (lifespan 120 days). The destruction of hemoglobin and the formation of bilirubin occurs in the cells of the phagocytic mononuclear cell system:
- Vysokovich-Kupffer cells, which capture and process old non-functional liver blood cells;
- splenic phagocytes - cells of the immune system that protect the body by absorbing harmful foreign particles or dying cells.
A brief scheme for the formation of bilirubin looks like this:
Red blood cells (in the spleen) - Hemoglobin (in the spleen) - Verdoglobin (a product of the enzymatic oxidation of the non-protein part of hemoglobin, formed in the cells of the macrophage system) - Biliverdin (an intermediate product of the breakdown of hemoglobin).
Stages of formation and excretion of bilirubin:
- First phase: formation of free (indirect) bilirubin in the spleen and hepatocytes (liver tissue cells).
- The second phase: its entry into the blood and transfer by albumins (simple water-soluble proteins) into hepatocytes.
- Third phase: binding of free bilirubin with glucuronic acid in hepatocytes (formation of bound, or direct, bilirubin).
- Fourth phase: release of direct bilirubin by hepatocytes as part of bile into the bile capillary.
- Fifth phase: entry of bilirubin-diglucoronide (direct bilirubin) into the duodenum in the form of bile.
Bilirubin diglucoronide can undergo two transformations:
- In the large intestine, it is converted into stercobilinogen, a substance created by colonic bacteria. Stercobilinogen is yellow in color and is further excreted in the feces in the form of stercobilin (provides the normal brown color of stool).
- In the small intestine it is converted into urobilinogen, a colorless product of the reduction of bilirubin, formed under the action of small intestinal bacteria. It is excreted through the kidneys with urine in the form of urobilin, and also enters the portal vein system into the liver with subsequent destruction.
Pathology of bilirubin metabolism in viral hepatitis
Damage to hepatocytes leads to a weakening of the uptake of free bilirubin and disruption of the process of addition of glucuronic acid to it (phase 3). As a result, the content of free bilirubin in the blood increases. Impaired transport of conjugated bilirubin into the bile capillary (phase 4) leads to an increase in conjugated bilirubin in the blood. At the same time, intrahepatic cholestasis is observed, i.e., the level of both free and bound bilirubin in the blood increases, and there is more of the latter (the skin turns yellow).
Bound bilirubin (the urine becomes dark in color) and bile acids penetrate from the blood into the urine, which reduce the surface tension of the urine and cause it to foam easily. Little bound bilirubin enters the intestines - the stool becomes discolored. Due to intrahepatic cholestasis and impaired bile secretion, little bile enters the intestine. Since bile is necessary for the digestion of fatty foods and the absorption of fat-soluble vitamins, due to its lack, a lot of fat remains in the stool (steatorrhea), and the absorption of vitamin K is impaired. Due to the low level of vitamin K, the synthesis of prothrombin in the liver decreases, as a result, blood clotting worsens .
General pathogenetic mechanisms in the liver during hepatitis
Cytolysis syndrome (cytolytic syndrome) is a set of signs indicating a disruption of the liver due to a violation of the integrity of liver cells and the release of liver enzymes into the blood. The degree of increase in the activity of aminotransferases (enzymes that reflect the functionality of the human liver) indicates the severity of the cytolytic syndrome, but does not directly indicate the depth of the dysfunction of the organ.
There is an empirical de Ritis coefficient - AsAT/AlAT. It reflects the ratio of the activity of serum AST (aspartate aminotransferase) and ALT (alanine aminotransferase). The de Ritis coefficient approximately indicates the predominant damage to one or another parenchymal organ. For hepatitis, its value is less than 1.33. In a healthy person it is in the range of 0.91-1.75.
Cholestasis syndrome is characterized by a violation of the outflow of bile. It lingers in the intrahepatic bile ducts, causing the liver tissue to swell and swell. There is an accumulation of bilirubin, cholesterol, β-lipoproteins and alkaline phosphatase in the blood. Also characteristic is the appearance of cholemia, a pathological syndrome characterized by the accumulation of bile acids in the blood. Normally, the acid content in the blood is 5-25 mmol/liter. If their number increases, cholemia develops, which is accompanied by itching, scratching, damage to the central nervous system (CNS) and staining of the skin through bile (80% bile acids + bilirubin).
Mesenchymal-inflammatory syndrome - damage to the liver parenchyma, connective tissue stroma, reticuloendothelium. Clinically expressed by enlarged liver and spleen, increased body temperature, acute phase parameters, as well as the level of autoantibodies, thymol test, β and γ proteins.
Immunosuppressive syndrome (secondary immunodeficiency) is a temporary or permanent depression of the immune system that develops under the influence of certain chemical and physical influences on the body, as well as due to certain infectious processes.
Biliary dyskinesia syndrome is a violation of their motility. It may occur due to changes in the innervation of the biliary tract due to the relative predominance of the tone of the vagus or sympathetic nerve.
Hepatic cell failure is a pathological process in which massive death of liver cells occurs. This condition is characterized by a decrease in albumin (manifested by edema syndrome) and a decrease in prothrombin (manifested by lethargy, fatigue, nausea, vomiting, hemorrhagic rash) [2][3][5][7][9].
Territorial distribution
A study conducted in collaboration with WHO and published in 2021 in the Journal of Hepatology1 estimates that hepatitis D virus affects nearly 5% of people with chronic hepatitis B worldwide and approximately one in five cases of liver disease and cancer. liver disease in those infected with hepatitis B is associated with coinfection with hepatitis D. The study identified several geographic areas of high prevalence of hepatitis D, including Mongolia, the Republic of Moldova, and several countries in West and Central Africa.
How to protect yourself from hepatitis D?
Since there is no vaccine against hepatitis D yet, infection can be avoided using several rules. Since hepatitis is most often transmitted sexually, you should always use condoms with new partners. For prevention, you can be tested for the hepatitis virus at least once a year.
The golden rule, which everyone knows about since childhood, is that all hygiene items must be individual. Never use someone else's toothbrushes, washcloths or towels. Have a custom set of dishes, your own plates, cutlery and mugs.
Choose dentists, beauty salons and tattoo parlors with caution. The hepatitis virus can be transmitted even through a small wound. The infection is transmitted through blood, so it can easily be acquired through unsterilized instruments.
Symptoms
Acute hepatitis: Co-infection with hepatitis B and D viruses can lead to moderate to severe hepatitis and in some cases fulminant hepatitis, but complete recovery usually follows and chronic hepatitis D is rare (in less than 5% of cases). cases of acute hepatitis).
Superinfection: a person who already has chronic hepatitis B can become infected with the hepatitis D virus. Superinfection with hepatitis D against the background of chronic hepatitis B leads to the accelerated development of more severe forms of the disease in 70-90% of patients, regardless of age. Patients superinfected with hepatitis D develop cirrhosis nearly 10 years faster than patients infected with hepatitis B alone. Patients with cirrhosis due to hepatitis D virus are at increased risk of developing hepatocellular carcinoma. The reason why hepatitis D virus causes more severe disease and accelerated fibrosis compared with hepatitis B monoinfection remains unknown.
Testing for hepatitis and its treatment
To determine the presence of the hepatitis D virus, blood is donated early in the morning, on an empty stomach. The following types of results can be obtained during the study:
- IgG anti-HDV - indicates the presence of class G antibodies. They indicate a past infection or infection of the patient.
- The IgM anti-HDV indicator determines class M antibodies and reflects the reproduction of the virus in the human body.
- HDAg is a special marker indicating the presence of a virus.
- HDV-RNA is an indicator by which one can judge the presence and reproduction of the delta hepatitis virus.
Treatment of hepatitis D is carried out strictly in a hospital. The doctor must approach this process comprehensively, depending on the severity and stage of the disease. Symptomatic drugs and immunomodulators are added to the main treatment.
The end of acute hepatitis can lead to either recovery or a chronic form. Only if you follow all the doctor’s recommendations, proper diet, rest and work, emotional stress and, of course, take medications, you can achieve the mildest course of the disease and prolong life.
Who is at risk?
Carriers of chronic hepatitis B are at risk of contracting hepatitis D.
Individuals who are not immune to hepatitis B (i.e., have not had the disease or have not been vaccinated against hepatitis B) are at risk for contracting hepatitis B, which in turn is a risk factor for contracting hepatitis D.
Groups at highest risk of co-infection with hepatitis B and D viruses include indigenous peoples, people living with HIV, and people who inject drugs.
Patients on hemodialysis, men who have sex with men, and sex workers also appear to be at increased risk of co-infection.
Migration from countries with a high prevalence of hepatitis D to areas with a lower prevalence of hepatitis D may have a negative impact on the epidemiology of hepatitis D in the host country.
Viral Hepatitis D (Hepatitis D, Hepatitis Delta, Hepatitis delta virus, HDV)
Hepatitis
Jaundice
8844 December 16
IMPORTANT!
The information in this section cannot be used for self-diagnosis and self-treatment.
In case of pain or other exacerbation of the disease, diagnostic tests should be prescribed only by the attending physician. To make a diagnosis and properly prescribe treatment, you should contact your doctor. Viral hepatitis D: causes, symptoms, diagnosis and treatment methods.
Hepatitis D (delta agent) is a liver disease caused by the hepatitis delta virus. The discovery of this virus took place in the mid-70s of the last century and was associated with the identification of a new antigen in the nuclei of hepatocytes in patients with severe forms of hepatitis B. According to the World Health Organization, from 15 to 20 million people in the world suffer from hepatitis D - mostly adult population.
Hepatitis D is a viral liver disease that occurs in both acute and chronic forms, the development of which requires the presence of the hepatitis B virus. Without the presence of hepatitis B in the patient's body, infection with hepatitis D is impossible.
HDV is a defective RNA virus. The defectiveness lies in the fact that the delta virus does not have its own supercapsid (envelope) and the necessary set of enzymes, and for its reproduction it requires hepatitis B antigen (HBsAg). Thus, hepatitis D does not exist on its own, but only together with hepatitis B.
The hepatitis D virus directly damages liver cells. This differs from hepatitis B virus infection, in which much of the liver damage occurs due to the body's own immune system attempting to destroy the affected cells.
Causes of hepatitis D
Hepatitis D is a blood-borne infection, spread by contact with the blood or body fluids of an infected person.
The main routes of transmission of the hepatitis D virus:
- surgical interventions, blood transfusions;
- contact with blood or objects contaminated with the patient’s blood (razor, toothbrush, manicure accessories, medical instruments);
- during non-medical manipulations - injecting drug use, piercing, tattooing;
- unprotected sexual contacts;
- during childbirth - vertical transmission of the virus (from mother to child).
Patients with hemophilia and other conditions requiring regular transfusions constituted the main group of people infected in the 1980s.
However, over time, their share in the total number of infected people is gradually decreasing, which is associated with the introduction of mass vaccination of the population against hepatitis B and routine screening of blood products for HBsAg. The reason for the newly increased prevalence of HDV infection in recent years is most likely the migration of the population from disadvantaged and hepatitis-endemic delta regions, which is confirmed by the high level of HDV carriage among the non-indigenous population of developed countries.
Classification of the disease Coinfection
is simultaneous infection with hepatitis D and hepatitis B viruses.
Superinfection
– infection with the hepatitis D virus of a child with chronic hepatitis B or a carrier of HBsAg (latent form of HBV infection).
According to the nature of the course, hepatitis D is divided into acute and chronic.
Hepatitis D symptoms
People who are not immune to hepatitis B (that is, who have not previously had the disease or have not been vaccinated against hepatitis B) are at risk for contracting hepatitis B, which in turn is a risk factor for contracting hepatitis D.
Although the symptoms of hepatitis D are identical to those seen with other forms of viral hepatitis, the disease is more severe. The incubation period for hepatitis D ranges from 21 to 180 days, but may be shorter in cases of additional infection.
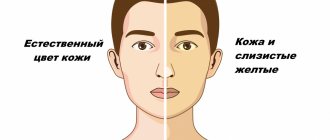
The pre-icteric period lasts from 4 to 10 days and is characterized by the following symptoms: increased fatigue, occasional rises in body temperature, loss of appetite, periodic pain in muscles and joints. The icteric period corresponds to more characteristic symptoms: yellowing of the skin, mucous membranes, whites of the eyes, darkening of urine, light-colored feces, nausea and vomiting, acute pain in the abdomen (in the area of the liver and stomach), itching. The icteric period lasts on average from two to six weeks.
There are two variants of acute hepatitis D, which differ significantly in course and outcome - coinfection and superinfection.
Coinfection occurs if a patient is simultaneously infected with hepatitis D virus and hepatitis B virus. In the vast majority of cases (more than 95%), the disease has a cyclical course and ends with spontaneous recovery, however, there is a higher level of malignant liver dysfunction and mortality than with infection alone hepatitis B virus.
Superinfection occurs when a patient with chronic hepatitis B virus infection becomes infected with hepatitis D virus. These patients typically experience a sudden deterioration in their condition, with approximately 90% of cases developing chronic hepatitis D, cirrhosis, and end-stage liver disease, making superinfection very dangerous.
Diagnosis of hepatitis D
The hepatitis D virus consists of an RNA molecule and a group of proteins. For the human body, these proteins are foreign molecules - antigens. In response to infection with the virus, the body begins to produce antibodies - anti-HDV.
HDV infection is diagnosed by detecting antibodies to the hepatitis D virus immunoglobulin G (IgG) and immunoglobulin M (IgM) classes, confirmed by detecting HDV RNA in blood serum using PCR.
- Hepatitis D virus, total antibodies. The analysis allows you to determine the presence of antibodies to the hepatitis D virus in the blood. Their presence will indicate both the presence of the virus in the body and the fact that the body is fighting the virus. Antibodies will also be detected in people who have been successfully treated for hepatitis D.
Screening and diagnosis
Diagnosis of hepatitis D is carried out by detecting a high concentration of antibodies to the hepatitis D virus of the class immunoglobulin G (IgG) and immunoglobulin M (IgM); To confirm the diagnosis, a test is carried out for the presence of hepatitis D virus RNA in the blood serum.
However, diagnostic tools for hepatitis D are poorly available, and hepatitis D virus RNA tests, which are used to monitor response to antiviral therapy, are not standardized.
If it is not possible to perform a quantitative test for hepatitis D virus RNA, it is advisable to conduct a quantitative test for HBsAg to monitor the response to therapy. A decrease in HBsAg titer often indicates disappearance of this surface antigen and clearance of the hepatitis D virus, although disappearance of surface antigen is rare in patients undergoing treatment.
Treatment
Current guidelines generally recommend pegylated interferon alfa for at least 48 weeks, regardless of response to treatment. The overall rate of sustained virological response is low, but this treatment is an independent factor associated with a lower likelihood of disease progression.
Current guidelines generally recommend pegylated interferon alfa for at least 48 weeks, regardless of response to treatment. With this treatment, the overall rate of sustained virological response is low, but its administration is an independent factor associated with a lower likelihood of disease progression. However, interferon treatment is associated with significant side effects and is contraindicated in patients with decompensated cirrhosis, active psychiatric diseases and autoimmune diseases.
More efforts are required to reduce the global burden of chronic hepatitis B and develop safe, effective and affordable hepatitis D drugs; this would ensure widespread access to treatment for those who urgently need it.
Symptoms
Hepatitis D is characterized by a very short incubation period when simultaneously infected with B (coinfection). In this case, the HDV delta virus begins to actively multiply already on the 4-5th day, aggravating the course of the liver infection.
The incubation period of superinfection - when the HDV delta virus “layers” on the already introduced hepatitis B virus - lasts from 3 weeks to one and a half to two months.
Common symptoms of hepatitis D for both types of infection are the following signs and conditions:
- change (yellowing) in the color of the skin, mucous membranes, whites of the eyes;
- change in urine color towards darker;
- significant lightening of stool;
- pain in the upper abdomen;
- intoxication of the body - nausea, vomiting;
- loss of appetite;
- general malaise and weakness, rapid fatigue;
- increased body temperature;
- muscle and joint pain.
Characteristic is the rapid development of the clinical picture and the rapid transition of the disease into a chronic form, which has common features with the chronicity of viral hepatitis of other origins:
- general malaise, fatigue, weakness;
- fever and chills in the absence of catarrhal manifestations;
- jaundice followed by abnormal redness of the skin;
- the appearance of spider veins;
- liver enlargement;
- liver edema, ascites.
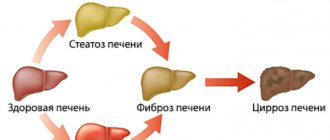
With chronic hepatitis D, periods of exacerbations and remissions alternate, and in the absence of adequate treatment, liver cirrhosis develops very quickly - the process of irreversible destruction of liver tissue with this type of infection takes only 1.5-2 years.
WHO activities
In May 2021, the World Health Assembly adopted the first Global Health Sector Strategy on Viral Hepatitis 2016–2021. The strategy emphasizes the critical role of universal health coverage and aligns its objectives with those of the 2030 Sustainable Development Goals. The strategy's overarching goal is to eliminate viral hepatitis as a public health problem and is reflected in global targets to reduce the number of new cases of viral hepatitis infection by 90% and reduce deaths from viral hepatitis by 65% by 2030. The strategy sets out the measures that countries and the WHO Secretariat must take to achieve these goals.
WHO has not published specific recommendations for hepatitis D; Effective means of preventing hepatitis D infection include prevention of hepatitis B transmission through vaccination, including timely administration of the first dose of vaccine immediately after birth, additional preventive antiviral therapy for pregnant women with appropriate indications, ensuring blood safety, maintaining injection safety when providing medical care, as well as health care services. reducing harm associated with injecting drug use, such as providing sterile needles and syringes. WHO is supporting Member States to scale up these evidence-based interventions.
In addition, to support countries in meeting the global hepatitis targets of the 2030 Agenda for Sustainable Development, WHO is working in the following areas:
- raising awareness, facilitating partnerships and mobilizing resources;
- developing evidence-based policy and collecting data to inform action;
- improving health equity in the hepatitis response;
- prevention of transmission of infection;
- expanding coverage of screening, care and treatment services.
In 2021, WHO is celebrating World Hepatitis Day under the theme “The fight against hepatitis can't wait” to highlight the urgency of eliminating hepatitis in order to achieve the goals by 2030. Key messages relate to the latest estimates of the burden of viral hepatitis and mortality from viral hepatitis at the global and regional levels, as well as the need to ensure certification of the elimination of hepatitis as a public health threat by 2030.