Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Quercetin belongs to the subgroup of flavonols and has an antioxidant effect due to its ability to suppress the process of lipid peroxidation. Quercetin reduces the concentration of toxic peroxidation and free radicals , stimulates (SOD) isoforms and catalase activity.
The anti-inflammatory and antiallergic effects of the drug are due to the ability of Corvitin to suppress the activity of hylauronidase , the synthesis of leukotrienes and calcium ATPase . The antitumor effect is based on the ability of the drug to inhibit the process of protein phosphorylation, as well as on its antioxidant and antiproliferative effects. Suppresses thrombogenesis , has a positive inotropic effect on the muscles of the ventricles of the heart (papillary muscle).
The antiarrhythmic effect is caused by suppression of oxidative stress and inhibition of leukotrienes . The cardioprotective effect is due to stimulation of the production of nitric oxide , which helps improve metabolism in the ischemic area. The drug improves the processes of restoration of microcirculation and blood circulation after a heart attack and stroke , restores vascular tone without pronounced changes. Quercetin promotes the growth of the immune system cell population ( lymphocytes , phagocytosis ), which reduces the manifestations of secondary immunodeficiency .
Pharmacokinetics
The drug after parenteral administration is quickly absorbed into the body. In the blood, the maximum concentration is reached after one minute. Quercetin forms a stable bond with plasma proteins. Penetrates through the BBB . Metabolized through the hepatobiliary system and excreted by the kidneys.
Pharmacological properties of the drug Corvitin
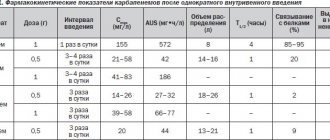
Pharmacodynamics . Quercetin, which is part of the drug, exhibits the properties of a modulator of the activity of various enzymes involved in the degradation of phospholipids (phospholipases, phosphogenases, cyclooxygenases), affecting free radical processes and responsible for the biosynthesis of nitric oxide, proteinases and others in cells. The inhibitory effect of quercetin on membranotropic enzymes and primarily on 5-lipoxygenase affects the inhibition of the synthesis of leukotrienes LTC4 and LTB4. Along with this, quercetin dose-dependently increases the level of nitric oxide in endothelial cells, which explains its cardioprotective effect in ischemic and reperfusion injury of the myocardium. It also exhibits antioxidant and immunomodulatory properties, reduces the production of cytotoxic superoxide anion, normalizes the activation of the subpopulation of lymphocytes and reduces the level of their activation. By inhibiting the production of anti-inflammatory cytokines interleukins IL-1β and IL-8, it helps to reduce the volume of necrotic myocardium and enhance reparative processes. The mechanism of protective action is also associated with preventing an increase in the concentration of intracellular calcium in platelets and activation of aggregation with inhibition of thrombogenesis processes. The drug restores regional blood circulation and microcirculation without noticeable changes in vascular tone, increasing microvascular reactivity. Pharmacokinetics. With a single intravenous infusion, the concentration of quercetin in the blood plasma rapidly increases. After administration of Corvitin at a dose of 10 mg/kg body weight, the maximum concentration of quercetin is noted 50 s after the start of administration; it is 123 μg/ml, of which 75 μg/ml is in the cellular elements of the blood and 48 μg/ml in the blood plasma. The half-life of Corvitin from whole blood, plasma and blood cells is 0.73±0.03, respectively; 0.85±0.01; 0.77±0.02 min. Along with the rapid metabolism of quercetin in the body, a long-term pharmacological effect is noted. The prolonged pharmacological effect of the drug may be associated with pharmacologically active metabolites, in particular chalcone, which significantly increases the fluidity of cell membrane lipids. After administration of the drug, the binding of quercetin to blood plasma proteins is more than 98%. It quickly spreads through the bloodstream and penetrates from the blood into the tissues. Quercetin penetrates the BBB and is metabolized in the liver. It is excreted primarily in the urine in the form of metabolites.
Corvitin, instructions for use (Method and dosage)
The drug Corvitin is diluted with isotonic NaCl (0.9%). It is necessary to introduce 50 ml of the solution and shake the bottle until the powder is completely dissolved.
In case of acute myocardial infarction, Corvitin is administered intravenously on the first day at a dosage of 0.5 g of the drug, immediately immediately after hospitalization, then after 2 hours and a subsequent dose after 12 hours. Introduce slowly over 15–20 minutes. During the second and third days, the drug is administered at a dosage of 0.5 g twice a day with an interval of 12 hours. During the fourth and fifth days - once a day at a dose of 0.25 g.
For acute stroke , Corvitin is administered in a dosage of 0.5 g intravenously after hospitalization after 2 and 12 hours. During the second and third days, the drug is administered at a dosage of 0.5 g twice a day every 12 hours. During the fourth to tenth day, once a day at a dose of 0.5 g.
Journal "Emergency Medicine" 6(31) 2010
The problem of treating acute ischemic stroke remains relevant in angioneurology [6]. The mortality rate from stroke in Ukraine exceeds the corresponding indicators in European countries and tends to further increase. Less than 20% of stroke survivors are able to return to their previous standard of living and work. The opportunity to correct hypoxia and ischemia missed in the early stages of therapy results in months and years of futile treatment. Patients with persistent focal neurological deficits require constant care from relatives, and patients with a persistent vegetative state become a heavy and hopeless burden for neurointensive care units [8].
According to the literature and our data obtained from a retrospective analysis of the quality of medical care for patients with acute stroke, only 15% of patients (for various reasons) are delivered in the first 6 hours, when the possibilities of thrombolysis have already been missed. Thus, in addition to organizational work with the ambulance service, it is necessary to optimize drug approaches to protect the brain within 6–12 hours from the onset of the disease (during the delivery time of the vast majority of patients). Neuroprotective therapy has a more complex mechanism of action compared to thrombolysis. The use of neuroprotectors ensures an increase in the duration of the survival period of neurons under ischemic conditions; with timely use of neuroprotection, inhibition of the reaction mechanisms of the ischemic cascade can be achieved. Strategies for rational neuroprotection are based on the activation of nonspecific resistance to hypoxia and consist of restoring adequate tissue perfusion and eliminating the toxic effects of hypoxia [4].
To protect the brain under conditions of ischemia, a large number of pharmacological drugs are used (nootropil, Mexidol, Ceraxon, Actovegin, Cerebrolysin, Semax and others), which have different points of application and have a variety of effects, united under the general concept of “cerebroprotectors” [3].
The debate regarding the appropriateness of neuroprotective therapy is currently one of the most heated [1, 4, 7]. Under experimental conditions, the cerebroprotective effect of many drugs that are used in the neurovascular departments’ own protocols has been demonstrated, but work continues to study the effectiveness and safety in randomized controlled trials (in some cases with negative or questionable findings) [8]. The specificity of damage to the brain substance dictates the need to take into account the multicomponent pathogenetic mechanisms of the development of cerebral stroke [6]. Taking this into account, it is necessary to search for drugs with multimodal effects, which will expand the choice and possibilities of treating cerebral stroke.
Such drugs include bioflavonoids, which allow them to immediately influence various parts of the pathobiochemical process. Bioflavonoids are an important component of antioxidant protection. Antioxidant properties are realized due to the ability to reversibly oxidize, the ability to intensify lipid peroxidation reactions, the ability to selectively absorb light energy of a certain wavelength, absorb and neutralize free radicals. Also, bioflavonoids effectively affect leukocyte hemostasis - they prevent blockade of microcirculation vessels by leukocyte plugs, suppress the synthesis of leukotrienes from arachidonic acid; reduce capillary permeability during anaphylactic shock, anaphylaxis; reduce the aggregation and adhesive properties of blood due to increased deformability or fluidity of the membranes of blood cells; exhibit endotheliotropic properties due to the ability to integrate into the membranes of endothelial cells, thereby protecting them from damage, increasing the synthesis of prostacyclin, nitric oxide while simultaneously reducing the production of thromboxane A2, endothelin 1; have an anti-edematous effect due to the restoration of endothelial cell function, stabilization of vascular permeability, which promotes adequate tissue perfusion; have a weak antispasmodic effect on the arteries and increase the tone of the veins [5]. The brain, especially under conditions of ischemia, is characterized by a low content of the main components of antioxidant defense, which explains its special sensitivity to the production of free radical oxidation. In cerebral stroke, bioflavonoids, due to their multimodal antioxidant properties, offer hope for effective neuroprotection. One of the representatives of bioflavonoids is Corvitin.
Experiments and clinical trials have demonstrated many interesting mechanisms of Corvitin's influence, thanks to which the drug has occupied a worthy niche as a cardioprotector for the treatment of acute coronary syndrome and myocardial infarction; as an angioprotector (for the treatment and prevention of reperfusion syndrome). Its cerebroprotective and nephroprotective properties are also discussed.
An important factor in the therapeutic effect is the fairly rapid introduction of the powerful antioxidant quercetin into the patient’s body. In acute ischemic stroke, Corvitin, due to its multimodal antioxidant properties, gives hope for effective neuroprotection. All this served as the motivation for this study.
The study falls into the category of open, randomized, comparative, parallel. The drug under study is Corvitin (lyophilized powder for the preparation of solution for injection) produced by JSC Scientific and Production Center "Borshchagovsky Chemical Plant". This clinical trial was conducted in accordance with the principles of Good Clinical Practice (GCP), the principles of the Declaration of Helsinki and the current legislation of Ukraine in the field of clinical research. The study was conducted as a multicenter study at three clinical sites: I - Oleksandrovsk City Clinical Hospital (principal investigator - Prof. Vinichuk S.M.), II - Kiev Emergency Hospital (principal investigator - Prof. Zozulya I.S.), III - Kharkov Hospital of Emergency and Emergency Medical Care named after. prof. A.I. Meshchaninova (responsible researcher - Prof. Nikonov V.V.).
The study was conducted according to a single protocol at all clinical sites.
The purpose of the study was to study the effectiveness and tolerability of Corvitin in patients with acute ischemic stroke. The study included patients with acute ischemic stroke (time from the onset of symptoms no more than 12 hours) aged from 18 to 65 years with a diagnosis confirmed by magnetic resonance/spiral computed tomography, with an NIHSS score of 8 to 15 points. In all cases, written informed consent was obtained from the patient and/or legal representative to participate in the study.
Material and methods
The study involved 198 patients with acute ischemic stroke. Among the examined women there were 81 (40.64%) and men - 117 (59.7%).
In the main group, patients received standard therapy and Corvitin according to one of three regimens:
1. Corvitin for 5 days (1st day - 1.5 g, 2nd - 3rd day - 1 g, 4-5th day - 0.5 g).
2. Corvitin for 10 days (day 1 - 1.5 g, days 2-3 - 1 g/day, days 4-10 - 0.5 g/day);
3. Corvitin for 10 days (day 1 - 1.5 g, days 2–10 - 1 g/day).
For intravenous administration of a dose of Corvitin in the amount of 0.5 g, the contents of the bottle were dissolved in 50 ml of isotonic sodium chloride solution. The solution was administered over 15–20 minutes.
In the comparison group, patients received only standard therapy (anticoagulants, antiplatelet agents, peripheral vasodilators, infusion therapy). After an interim report, treatment regimen 1 was excluded due to lack of effectiveness. The structure of the surveyed groups is shown in Fig. 1.
Patients in both groups were observed from the 1st to the 21st day of stroke (inpatient period), then outpatient observation (outpatient period) was carried out from the 22nd to 120th day from the onset of the disease (aspirin 100 mg/day daily, dipyridamole at 25 mg 2 times a day daily, pentoxifylline 0.1 g 3 times a day).
Analysis of individual registration forms of patients included in the study indicated a high degree of homogeneity of patient groups and representativeness of the study. Up to 60% of patients were delivered within 6 hours (Fig. 2).
It is noteworthy that 83.3% of the patients included in the study had hypertension of varying degrees as one of the factors in the development of cerebral catastrophe (Fig. 3). 23.66% of patients suffered from overweight of varying severity, 19.35% of patients had cerebral atherosclerosis. About 4.84% of patients had diseases of the respiratory system (chronic obstructive pulmonary disease - COPD or bronchitis). 11.29% of patients suffered from heart failure, and 7.53% of patients suffered from atrial fibrillation.
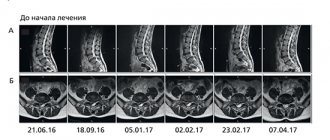
MRI/SCT of the brain was performed on the 1st day of the disease - before the start of therapy. Tomograms determined the presence and location of the brain lesion. In the absence of an ischemic lesion during screening, a repeat study was carried out in the period from 3 to 7 days. Neuroimaging data revealed similar localization of the ischemic lesion in both groups (Fig. 4) with a predominance of lesions in the left carotid system, which may indicate a probably greater severity of neurological deficit (including due to aphasic disorders).
The National Institutes of Health Stroke Scale (NIHSS) was used to objectify the severity of the condition, the severity of focal neurological deficits and assess the dynamics of clinical indicators. The degree of functional recovery was determined using the modified Rankin scale. Neurological deficit and the degree of functional recovery were assessed upon admission (during screening), as well as on days 6, 11, 21, 60, 90, and 120 of the disease.
On the first day of stroke upon admission, an ECG was recorded in all patients to exclude acute cardiac pathology. Laboratory parameters (blood test, urine test, biochemical blood test) were performed on days 1, 11, and 21 of the disease.
Considering the key role of cytokines in the development and outcome of ischemic stroke, the initiation of processes leading to damage to brain tissue, the dynamics of inflammatory markers - neurospecific proteins (protein S-100, GFAP, NSE) in patients at the II clinical base, as well as the dynamics of indicators of inflammatory markers (CRP, interleukin-6) on days 1, 3, 10 at clinical bases I and II; dynamics of inflammatory markers (interleukin-6, -10) on days 1, 10, 21 at clinical bases I and II.
From the position of oxidative stress as an early manifestation of the pathophysiological ischemic cascade, the dynamics of lipid peroxidation indicators (SOD, MDA, DC) were determined in both groups on days 1, 5, 10, 21 at clinical bases I and II.
Considering that the main cause of stroke is circulatory disturbance in the main arteries of the head, it was important to evaluate the effect of Corvitin on the hemodynamic parameters of the intracerebral vessels of the brain using transcranial Doppler sonography on the 1st, 10th, 21st days of the disease.
The effectiveness criterion was a decrease in the sum of scores for assessing the neurological status of patients according to the NIHSS scale, and a decrease in scores for assessing the degree of disability on the modified Rankin scale.
Tolerability assessment was carried out on the basis of subjective symptoms and objective data obtained during treatment, dynamics of laboratory parameters, as well as the frequency of occurrence and nature of adverse reactions/events. Mortality rates and adverse reactions/events were monitored for 120 days.
The effectiveness of therapy was assessed after statistical processing of individual registration forms using the Statistica for Windows software package (version 5.5).
Results and discussion
The analysis showed that the neurological disorders of patients with acute ischemic stroke in the main group and the comparison group corresponded to moderate and severe stroke.
Observation of the examined patients revealed a significant regression of the initial neurological and general somatic symptoms. In patients with a degree of depression of consciousness from stupor to stupor, already at the first administrations of Corvitin, an “awakening” effect was observed: patients were more willing to engage in speech contact, improvement in motor activity was noted, patients responded better to changes in body position and to external stimuli. Patients who received Corvitin, according to their caring relatives, had more active contact with relatives and participated in physical therapy activities. In patients receiving standard therapy, a clear level of consciousness was restored more slowly; they were more apathetic and asthenic.
During treatment with Corvitin, all patients to one degree or another, significantly more often when using regimen 3, already after the first infusions of Corvitin noted an improvement in general well-being: complaints of headache, dizziness, fatigue decreased or disappeared, there was improved sleep, a decrease in tinnitus and etc. All this had a positive effect on the emotional status of patients, in contrast to patients in the comparison group (p < 0.05). The patients’ great adherence to therapy was noteworthy; during rehabilitation courses, patients asked to be prescribed Corvitin exclusively in complex therapy.
Analysis of clinical data showed that the most noticeable clinical effect was observed in the main group starting from the 6th day of the disease (in the comparison group - from the 11th day, p < 0.05) in relation to such manifestations as damage to the pyramidal tract, vestibular dysfunction . The effect on dysfunction of the pelvic organs, sensory and speech function, and extrapyramidal symptoms occurred somewhat more slowly. Sensory function improved statistically significantly starting from the 11th day of the disease in the main group, and from the 120th day in the group of patients receiving standard therapy. Summary data on the effect of Corvitin on individual clinical symptoms over the course of treatment are presented in Fig. 5 and 6.
Particular attention, given the initially highest incidence of left hemisphere infarcts in the main group (correspondingly, the greater severity of aphasic disorders), is the effect of Corvitin on the restoration of speech functions in stroke. To detail the dynamics of speech disorders, we used a speech questionnaire in 62 patients. The results showed that in patients receiving Corvitin, speech was restored better (statistically significant from the 11th day, in the comparison group - from the 21st day); this was manifested by an improvement in both speech production (expressive function) and understanding of addressed speech (impressive function). The patients’ speech became more intelligible, intelligible, and confident, and the pronunciation of both individual words and entire phrases improved. As a result, patients receiving Corvitin had an improved prognosis for further successful rehabilitation.
When analyzing the dynamics of the neurological status according to the NIHSS scale (Fig. 7), a significant regression of focal neurological deficit was revealed in patients receiving Corvitin, starting from the 6th day of the disease (in the comparison group - from the 11th day), reaching an even greater contrast by 11 on the 90th and 21st days of the disease and remained at the achieved level by the 90th and 120th days after the stroke. The results obtained are encouraging in terms of the further prognosis of the patient’s recovery after a stroke.
The background level of disability in both groups according to the modified Rankin scale in almost all patients corresponded to a pronounced degree of disability: inability to walk without assistance and cope with one’s physical needs without assistance (i.e., compliance with the level of “severe dependence on external assistance”). It is encouraging that in the group of patients receiving Corvitin, a significant (p < 0.05) decrease in the score on the modified Rankin scale was established. An analysis of the dynamics of the average values of assessments of the degree of disability of patients on the Rankin scale is presented in Fig. 8.
Statistical analysis showed that by the 6th day of stroke in patients receiving Corvitin, the level of disability corresponded to a “moderate degree of disability” - the patient requires assistance, but moves independently. In the main group, on the 11th day, a significant dynamics of reduction in disability was noted, in contrast to the comparison group, where a statistically significant decrease was observed by the 60–90th day of stroke.
The distribution of patients according to the degree of disability by the 120th day is indicative (Fig. 9).
According to the results of a qualitative analysis of ratings on the Rankin scale, the NIHSS scale (Fig. 9, 10), if we consider the sum of the proportions of patients belonging to the categories “no symptoms” and “no significant disability”, then the proportion of such patients in the main group was 78.18 %, and in the control group - 61.82%. These differences in proportions indicate that Corvitin significantly (p < 0.05) increases the likelihood of complete recovery in patients who have suffered a stroke. Thus, an assessment of the dynamics of recovery of focal neurological deficits revealed reliably confirmed neuroprotective properties of Corvitin (complete recovery was achieved after 120 days in 78.18% of patients). The quality of life of patients taking Corvitin improves earlier compared to the quality of life of patients on standard therapy.
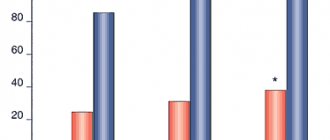
When assessing the overall effectiveness in the study groups (Fig. 11), it was noted that the use of Corvitin was more effective: high efficiency was recorded from the first day, reaching a maximum by the 21st day (2nd scheme - 78.33%, 3rd scheme - 77.97% of patients), and remained at the achieved level during the remaining two months of the outpatient observation period (81.67 and 88.14%, respectively). In patients receiving standard therapy, high effectiveness occurred later - from the 21st day of stroke and amounted to 56.14%, and on the 120th day it did not achieve the results of treatment in the main group (75.44%).
The study determined indicators of lipid peroxidation: superoxide dismutase (SOD) activity, malondialdehyde (MDA) and diene conjugate (DC) content in blood plasma and erythrocytes. Changes in MDA, SOD and DC parameters were small, but this fact itself sufficiently indicates the activation of lipid peroxidation products. In the dynamics of treatment with Corvitin, the tension of antioxidant defense was balanced, which may indicate the antioxidant properties of Corvitin, as well as an improvement in collateral blood circulation and microcirculation.
The therapeutic effect of Corvitin was also manifested in the inducing effect on the activity of the enzymatic component of antioxidant protection: normalization of DC values was statistically significant by the 10th day of the disease (in the comparison group, statistically insignificant by the 21st day). At the same time, by the 21st day of stroke, with the use of Corvitin, there was an increase, although not significant, in the activity of enzymes of the antioxidant system - the intracellular enzyme SOD. Thus, the proportion of patients with normal values of this enzyme increased for the 2nd treatment regimen from 55 to 60%, for the 3rd treatment regimen - from 80 to 82%, which may indicate greater effectiveness of the 3rd treatment regimen and proves the influence of Corvitin on free radical processes in brain tissue. Among patients receiving standard therapy, the proportion of patients with normal levels of this enzyme decreased by day 21 from 81 to 80%.
Interesting remark by S.M. Vinichuk et al. [10] that the antioxidant properties of Corvitin cannot be assessed by the intracellular enzyme SOD alone, when glutathione enzymes play the main role in antioxidant protection; the need for additional clinical and biochemical studies in studying the antioxidant properties of Corvitin with determination of the level of enzymes of the glutathione system is substantiated.
As is known, one of the early clinical manifestations of acute stroke is an increase in the activity of proinflammatory cytokines and acute phase proteins. For this purpose, the following indicators were determined: protein S100, glial fibrillary acidic protein (GFAP), neuron-specific enolase (NSE), C-reactive protein (CRP), interleukin-6, -10. Well-balanced interactions between the brain and the immune system are disrupted in acute ischemic stroke. IL-6 is a predictor of the development of cardiovascular complications. Our study revealed a deficiency of both cytokines against the background of signs of autoimmune inflammation, as evidenced by an increase in the concentration of CRP. This apparently indicates a decrease in the body's defenses.
Determination of the level of markers of neuronal and glial damage (NSE) and S100 protein to characterize the severity of neurological disorders in ischemic stroke and is used to assess its prognosis. The study did not reveal significant correlations between indicators of neuronal and glial damage and the severity of stroke, which was apparently due to the small sample of patients.
The use of Corvitin increased cerebral perfusion by improving the hemodynamic properties of blood. The results of a comprehensive analysis of the effect of Corvitin on cerebral blood flow in patients with ischemic stroke showed a tendency to improve cerebral blood flow indicators in the form of an increase in its linear velocity, a decrease in its deficit, and an increase in interhemispheric connections (reliably significant by the 10th day, reaching a maximum value by the 21st day diseases (p < 0.05)). At the same time, statistically significant differences remained in the restoration of cerebral circulation between the main group and the comparison group. Under the influence of Corvitin, when using the 3rd treatment regimen, a significant increase in the linear velocity of blood flow in the basilar artery was observed from 34 ± 2.42 cm/s to 46.70 ± 1.24 cm/s by the 10th day. Attention is also drawn to the positive effect of Corvitin on the dynamics of hemodynamic parameters in the vessels of the vertebrobasilar region with damage to the carotid system, which may indicate the mobilization of hemodynamic reserve and normalization of hemorheological factors.
Based on clinical and statistical analysis of subjective symptoms and objective data obtained during treatment, the dynamics of laboratory parameters, as well as the frequency and nature of adverse reactions/events, it was concluded that Corvitin was well tolerated in patients with acute ischemic stroke.
The occurrence of PR/PY was monitored throughout the clinical study. All AEs/AEs were not related to Corvitin treatment.
Against the background of the applied treatment regimens for acute ischemic stroke, serious side effects occurred in 6 patients - a fatal outcome, which amounted to 3.06% (6/198) of the total number of patients examined. Early death within the first 14 days occurred in 3 patients, in 1 patient - on the 22nd day, in two more observations - on the 57th and 66th days of observation. During treatment with Corvitin, a fatal outcome occurred in 4 patients out of 138 (2.90%), and with standard therapy - in 2 patients out of 60 (3.33%). A detailed analysis of fatal cases showed that deaths were not associated with the use of the study drug Corvitin, but were caused by severe stroke, the presence of extensive ischemic foci, concomitant pathology (coronary artery disease, atrial fibrillation, metabolic syndrome), and complications of the respiratory system (bronchopneumonia).
Of the non-serious side effects, two cases (1.45%) of a short-term decrease in blood pressure (BP) were recorded, which occurred in patients during the use of the 3rd treatment regimen and did not require additional therapy.
Analysis of side effects showed that the treatment methods used did not differ significantly in terms of mortality. The incidence of other serious side effects occurring during the course of treatment was also insignificant and not related to taking the drug Corvitin. The cause-and-effect relationship between the development of non-serious side effects (arterial hypotension) when using the 3rd treatment regimen was also questionable.
Thus, the neuroprotective properties of Corvitin are provided by various mechanisms, primarily by powerful antioxidant ones, which provide neuron protection under ischemic conditions. To increase the effectiveness of treatment of acute ischemic stroke, it is necessary to use optimally justified doses of the drug (1.5 g on the 1st day of stroke, 1 g/day on days 2–10), which at the same time are safe for patients.
The most illustrative, from our point of view, will be some clinical observations of patients receiving Corvitin according to regimen 3.
Patient D., 57 years old, was admitted to the neurological department of the Kharkov City Clinical Hospital. Productive contact is not available due to total aphasia. According to the daughter and the SME doctor, he became acutely ill when a severe headache, numbness and weakness appeared in the right limbs, and speech was impaired. SME was delivered urgently 5 hours after the onset of the disease.
Taking into account clinical and neurological data, MRI of the brain made a diagnosis: ischemic stroke in the basin of the left middle cerebral artery with right-sided hemiplegia, total sensorimotor aphasia against the background of discirculatory hypertensive encephalopathy of the 2nd stage. Concomitant diagnosis: hypertension, stage III. IHD, post-infarction (of unknown duration) cardiosclerosis. CH ІІА st.
Objectively: normosthenic. Upon admission, the patient's condition was assessed as severe, 15 points on the NIHSS scale, 5 points on the modified Rankin scale.
Taking into account the delivery of the patient within the “therapeutic window” and compliance with the inclusion criteria, the patient was taken into the study; Scheme 3 was chosen using the envelope method.
After 2.5 hours from the start of therapy, the patient showed signs of speech production; Sensory aphasia regressed, cephalgia was relieved. Subsequently, a good rate of recovery of the focal neurological deficit was observed: by the 6th day, the score on the NIHSS scale was 5 points, on the Rankin scale 2 points. Neurological status data correlated with neuroimaging data - there was a significant decrease in perifocal edema of the infarct area by the 6th day of the disease. The patient had a good mood and actively participated in all rehabilitation activities. The patient was rehabilitated by the end of observation to the level of mild disability - he copes with everyday activities without assistance, but is not able to perform some of his previous duties.
The following observation demonstrates the effectiveness of the use of Corvitin for stroke in the vertebrobasilar region, and fairly rapid relief of symptoms of vestibular dysfunction.
Patient Ch., 55 years old, was taken to the neurological department of the Kharkov City Clinical Hospital for Emergency Medicine by the MSP team with complaints of severe systemic dizziness, nausea, and repeated vomiting. SME was delivered urgently 3 hours after the onset of the disease.
Taking into account clinical and neurological data, MRI of the brain made a diagnosis: ischemic stroke in the vertebrobasilar region with pronounced vestibuloatactic and asthenoneurotic syndromes. Concomitant diagnosis: hypertension, stage III. Objectively: normosthenic. Upon admission, the patient's condition was assessed as severe, the NIHSS score was 10 points, and the modified Rankin scale was 5 points. The patient behaved extremely excitedly, aggressively, and angrily upon admission, shouting: “I’m paralyzed!”, “I’m going to die!”, she experienced repeated vomiting when opening her eyes, with the slightest change in body position.
After 5 hours from the start of therapy, the patient stopped vomiting, she became calmer, and the intensity of dizziness decreased somewhat. On the 5th day from the onset of the stroke, the patient experienced complete regression of systemic dizziness, elements of vestibular dysfunction persisted, and the patient sat up in bed independently. Subsequently, positive dynamics of recovery of focal neurological deficit were observed: by the 10th day, the score on the NIHSS scale was 2 points, on the Rankin scale 1 point. MRI of the brain did not reveal any ischemic focus. According to the patient and her relatives, the patient felt better on the first day of treatment (“Corvitin increased my vitality”), and the patient actively participated in all rehabilitation activities.
In a patient with psychosomatic personality characteristics, the use of Corvitin in complex therapy made it possible to normalize the functioning of the central nervous system by normalizing the irritability process and increasing resistance to stress in conditions of acute vestibular dysfunction. The patient was rehabilitated by the end of observation to the level of “no symptoms” and returned to her previous place of work (school teacher).
From our point of view, a certain role in the effectiveness of treatment is played by the fact that the patients who were in the study in the main group expect healing from the use of Corvitin. And this is understandable - the doctor-researcher regularly (according to the telephone contact schedule) called the patient, inquired about his well-being, detailed complaints, invited him for an examination, and provided the whole range of examinations. Thus, stroke patients feel care, attention, and interest in their problems, which is very important for older people in general and those who have suffered an acute stroke in particular and once again emphasizes the importance of deontology in the supervision of a stroke patient. Deontological problems of the relationship “doctor – stroke patient – society”, unfortunately, remain relevant at the present time along with the search for effective and safe neuroprotectors.
conclusions
1. Corvitin is a reliably effective pathogenetic drug in the intensive care of patients with acute ischemic stroke.
2. Recommended regimens for using the drug:
— Corvitin for 10 days: 1st day — 1.5 g (upon admission 0.5 g, after 2 hours 0.5 g, after 12 hours 0.5 g), 2nd — 3rd day — 1 g/day (with an interval of 12 hours, 0.5 g), days 4–10 - 0.5 g/day;
— Corvitin for 10 days: 1st day — 1.5 g (upon admission 0.5 g, after 2 hours 0.5 g, after 12 hours 0.5 g), days 2–10 — 1 g /day (0.5 g at intervals of 12 hours).
For intravenous administration of a dose of Corvitin in the amount of 0.5 g, dissolve the contents of the bottle in 50 ml of isotonic sodium chloride solution. The solution is administered over 15–20 minutes.
3. Therapy with Corvitin provides the possibility of faster and more complete functional recovery of patients with ischemic stroke, as well as an increase in the number of patients with complete recovery by the end of the acute period of stroke to 78.18% (with standard therapy - 61.82% of patients).
4. Corvitin significantly improves the quality of life of patients.
5. When using Corvitin, subjective symptoms decreased significantly more often, the emotional state of patients improved, and there was a greater adherence of patients to therapy.
6. Corvitin is a safe drug.
7. It is necessary to note the convenience of titrating the dose of Corvitin, the possibility of increasing the dose to 1.5–2 g in severe cases of cerebral stroke without harm to health (adverse reactions and events were not associated with large doses of the drug).
8. Corvitin is a good basis for combination with other necessary intensive care drugs (hypotensive, antiarrhythmic, hypoglycemic, anticoagulants, etc.), which will ensure the safety of therapy for cerebral stroke.
These studies allow us to express an opinion about the need for further in-depth studies of the positive effects of Corvitin when used as undifferentiated basic therapy (regardless of the type of stroke), as well as the advisability of early use (from the prehospital stage).
Thus, the study confirmed the effectiveness of the multimodal drug Corvitin and its pronounced neuroprotective effect. Corvitin can be recommended in the complex treatment of acute ischemic stroke (ideally, with inclusion in the Standard of Care for a Patient with Acute Ischemic Stroke).
Interaction
Do not dissolve the drug in a solution of dextrose and rheopolyglucin . When taking Corvitin together with nitrates , the hypotensive effect of nitrates is enhanced. Corvitin, when taken together with fibrinolytics , increases the risk of thrombolytic complications. Corvitin is pharmacologically incompatible with other solutions and drugs. The drug can be combined with antithrombotic, antiarrhythmic, antianginal and fibrinolytic drugs.
Side effects of the drug Corvitin
The drug is well tolerated by patients. However, with rapid intravenous administration or in combination with organic nitrates, temporary moderate arterial hypotension may occur. Isolated cases of the following adverse reactions have also been noted: from the central nervous system : dizziness, headache, numbness of the tongue, tremor, chills, tinnitus, agitation or general weakness; allergic reactions : urticarial rash, urticaria, itching, anaphylactic shock; other : facial flushing, chest pain, difficulty breathing.
Corvitin's analogues
Level 4 ATC code matches:
Aescusan 20
Aescusan
Venitan
Venen gel
L-Lysine escinate
Dihydroquercetin
Endothelon
Antithromb , Doxy , Quercetin , Escuvit , Calcium dobesylate , Endotelon , Cyclo 3 Fort .
Reviews of Corvitin
According to doctors, the inclusion of Corvitin in the treatment regimen for myocardial infarction in patients with symptoms of acute heart failure helps to normalize the pumping function of the heart and reduces dilatation of the left ventricular cavity. A particularly pronounced effect was noted on the first day of acute myocardial infarction in patients with dysfunction of the left ventricular muscle. The use of the drug in the early period of the disease helps to reduce the mass of necrotic myocardium .
The drug is also effective in intensive therapy of patients with acute ischemic stroke . Its administration makes it possible to achieve a more complete and relatively rapid functional recovery of such patients, and also increases the rate of complete recovery of patients by the end of the acute period of stroke . Taking the drug helps reduce subjective symptoms, as well as improve the emotional state of patients.
Contraindications
- Individual sensitivity to quercetin and/or other components of the drug;
- severe arterial hypotension.
hypersensitivity to drugs with P-vitamin activity;
Interaction with other drugs and other types of interactions.
In combination with organic nitrates
Corvitin® may cause arterial hypotension.
The simultaneous use of the drug with fibrinolytics
leads to an increase in the effectiveness of thrombolytic therapy.
Do not use solutions of glucose, rheopolyglucin and other solutions as a solvent for the drug Corvitin®.
The drug is used in combination with antianginal, antiarrhythmic, antiplatelet drugs
and
fibrinolytic
agents.
When using the drug:
- with ascorbic acid
– a summation of effects is observed; - with non-steroidal anti-inflammatory drugs
– the anti-inflammatory effect of the latter is enhanced while the ulcerogenic effect is reduced; - with digoxin
– the maximum concentration in the blood serum and the total area under the concentration-time curve of digoxin increases; - with cyclosporine
– the bioavailability and concentration of cyclosporine in the blood increases; - with paclitaxel
– effect on the metabolism of the latter; - with verapamil
– the bioavailability of the latter increases; - with tamoxifen
- bioavailability increases, metabolism and excretion of the latter decreases.
Corvitin price, where to buy
The price of lyophilized Corvitin powder in 0.5 g bottles No. 5 averages 1,250 rubles per package. You can buy Corvitin in most pharmacies in Moscow and other cities only by pre-order, or you will be offered to purchase analogues of the drug.
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
Pharmacy24
- Corvitin 0.5 g No. 5 powder PAT NVC "Borshchagivsky chemical-pharmaceutical plant", Kiev, Ukraine
555 UAH. order
PaniPharmacy
- Corvitin bottle Corvitin pores. liof. d/in. 0.5g No. 5 Ukraine, Borshchagovsky Chemical Plant PJSC
549 UAH. order
show more
Compound
active substance:
corvitin, which is a complex of quercetin and povidone;
1 bottle contains corvitin, which is a complex of quercetin with povidone, - 0.5 g and is produced according to the recipe: quercetin (in terms of 100% dry matter) - 0.05 g, povidone with a molecular weight of 7100-11000 (in terms of anhydrous substance) – 0.45 g;
excipient: sodium hydroxide.
Dosage form.
Lyophilisate for solution for injection.
Basic physical and chemical properties:
dry porous mass from light yellow to yellow with a greenish tint, hygroscopic.
Pharmacotherapeutic group.
Capillary stabilizing agents.
Code ATX С05С Х.