Causes
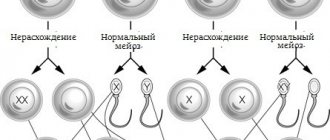
If there is no family history of this disease, and the parents are in absolute health, there is still a risk of having a child with this disease. Science knows that a human cell contains 46 chromosomes. The egg and sperm contain twenty-three chromosomes. When they combine, the number of chromosomes also combines. The causes of Edwards syndrome are not well understood to date.
Researchers say that due to gene mutations, an extra one is formed in the 18th pair of chromosomes. In 2 cases of this disease out of 100, the eighteenth chromosome is lengthened, while the 47th chromosome is not formed, but a translocation occurs.
In three out of a hundred cases of the disease, doctors talk about mosaic trisomy. This means that the extra chromosome is present only in some cells of the fetal body, and not in absolutely all. But in terms of symptoms, the three described variants of the disease converge. Only in the first case can the course be severe and there is a greater chance of death.
Trisomy 18 (Edwards syndrome) is the most common autosomal abnormality in live births after Down syndrome (trisomy 21). In most cases, trisomy 18 (Edwards syndrome) is true, i.e. its cause is the nondisjunction of chromosomes during meiosis. In a small number of cases (up to 10%), trisomy 18 (Edwards syndrome) is mosaic (mosaicism is a condition in which the body contains tissues of genetically different types, for example, normal cells and cells with three chromosomes), caused by postzygotic chromosome nondisjunction in anaphase - at an early stage of embryo development (Chen 2004, Forrester 1999, Carothers 1999, Huether 1996, Pradat 1991, Buyse 1990. Partial trisomy is also possible. In this case, part of chromosome 18 joins another chromosome. This effect is called “Translocation”, and it can occur both during the maturation of gametes and after fertilization in the cells of the embryo. In the cells of the body, there are two homologous chromosomes 18 and, in addition, a part of chromosome 18 attached to another chromosome. In people suffering from partial trisomy 18, the anomalies appear weaker than with typical Edwards syndrome. Most trisomy 18 are detected in fetuses in the middle of the second trimester and very often such pregnancies end in intrauterine fetal death. (Hook, 1989).
Clinical features associated with trisomy 18 include, but are not limited to: central nervous system disorders (holoprosencephaly, meningomyelocele), eye, nose, cleft lip and/or palate malformations, auricular deformities, deformed limbs, polydactyly, and heart defects , genitals, etc.
The prognosis for this disease is usually unfavorable. Most babies born die, on average, within 2 to 10 days (Parker, 2003). However, there are children whose life expectancy reaches a year or more (Rasmussen, 2003). However, such children have both physical and mental developmental delays (Parter 2003). Population frequency is approximately 1:7000.
ETIOLOGY
The cause of true trisomy 18 is the nondisjunction of chromosomes during the formation of the egg and sperm, when one gamete receives an additional 18th chromosome. Nondisjunction can occur in the first (MI) or second (MII) meiotic stage.
In 90-97% of cases, the additional chromosome 18 is of maternal origin, and paternal origin in 3-10% of all cases. Among cases of maternal trisomy 18, 31–39% result from MI nondisjunction and 61–69% result from MII nondisjunction (Bugge et al., 1998; Nicolaidis and Petersen, 1998; Eggermann et al., 1996 ; Ramesh and Verma, 1996; Fisher et al., 1995; Jacobs and Hassold, 1995; Fisher et al., 1993; Ya-gang et al., 1993).
DEMOGRAPHY AND REPRODUCTIVITY
The risk of trisomy 18 is known to increase with maternal age (Munne 2004, Naguib 1999, Baty 1994, Buyse 1990, Goldstein 1988, Schreinemachers 1982). The risk of trisomy 18 (Edwards syndrome) is also associated with increasing paternal age, however, if the risk of trisomy 18 increases due to maternal age, then the paternal age is neglected in such cases. (Naguib 1999, Baty 1994). For women over 45 years of age, the risk of having a sick child is 0.7%.
Race/ethnicity has little effect on the risk of trisomy 18 (Buyse 1990). One study found that of four racial/ethnic groups examined (Caucasians, East Asians, Pacific Islanders, Filipinos), the risk of trisomy 18 was highest for Far East Asians and lowest for Pacific Islanders (Forrester, 1999). However, these differences in risk appear to be related to differences in the distribution of maternal age among these racial/ethnic groups.
Geographic area may influence the risk of trisomy 18. One study found that the risk of trisomy 18 was higher among urban residents (Forrester, 1999). This increased risk remains after controlling for maternal age. Several studies have shown that the prevalence of trisomy 18 may be influenced by seasonal variations (Naguib, 1999).
Some studies have shown that Trisomy 18 (Edwards syndrome) has been on the increase in recent years. However, this may be due to both improved diagnosis of aneuploidies, incl. prenatal diagnosis and diagnosis of fetuses (Pradat 1991), and with increasing age of women giving birth (Gessner 2003, Forrester 1999).
Over the past few decades, it has been established that the serum of women carrying fetuses with trisomy 18 contains low levels of alpha-fetoprotein, human chorionic gonadotropin, and estriol (anick 1993, Greenberg 1992, Doran 1986). In addition, prenatal ultrasound examination reveals various structural abnormalities often associated with trisomy 18 (Abramsky 1993, Vintzileos 1987). Prenatal screening of markers, ultrasound and a final diagnosis confirmed by karyotyping using procedures such as amniocentesis and chorionic villus sampling can determine trisomy 18 during fetal development.
The sex of the child affects the risk of trisomy 18. Girls with trisomy 18 are born 3 times more often than boys. (Forrester 1999, Naguib 1999, Carothers 1999, Huether 1996, Pradat 1991, Buyse 1990, Goldstein 1988). One study found that sex ratios varied across racial/ethnic groups, but these observations were based on small samples, making the relationship uncertain (Huether 1996).
The risk of recurrence for trisomy 18 is approximately 1% (Baty 1994, Buyse 1990). Recent studies have shown that the risk of trisomy increases in women who have had trisomy 18 in previous pregnancies, regardless of live birth or intrauterine fetal death. That is, even if the pregnancy ends spontaneously, the risk remains increased (Munne 2004). There is also an increased risk of trisomic pregnancy in women with low oocyte counts (Kline, 2000). This condition is likely associated with the onset of menopause.
LIFESTYLE AND ENVIRONMENTAL FACTORS.
There is no reliable evidence to support the influence of environmental factors on the risk of trisomy 18. However, differences in the prevalence of trisomy 18 between populations (Forrester 1999, Naguib 1999) suggest that environmental factors may influence the risk of chromosomal errors. However, there is no increased risk of trisomy 18 in people living near solid waste dumps or incinerators (Cordier 2004, Harrison 2003). The presence of chlorides and nitrates in drinking water (Cedergren 2002) and pesticides (Berkowitz 2003) do not increase the risk of this defect. Mothers whose work involves chemical solvents also do not increase the risk of trisomy 18 (Wennborg 2005).
The likelihood of chromosomal errors may now be increasing due to assisted reproductive technologies (ART). However, it is not entirely clear whether this is due to imperfect laboratory methods or to genetic disorders in the parents, which at the same time can also be the cause of infertility. That is, couples who cannot conceive naturally may be predisposed to genetic errors (Palermo 2000).
One study found that differences in folate metabolism in women are not associated with meiotic errors leading to aneuploidy.
Regular multivitamin use is not associated with a reduced risk of trisomy 18 (Botto 2004).
GENETIC PRESPOSITION
Sometimes, due to a phenomenon called balanced translocation, some people may carry genetic material belonging to chromosome 18 on another chromosome. Because such people do not have additional genetic material, they do not have signs of trisomy 18. However, such people have an increased risk of having children with the condition. Carriage of a balanced chromosomal translocation can be determined by studying the karyotype.
In rare cases, one parent may be a carrier of partial trisomy 18, which can be inherited. The carriage of partial trisomy can be determined using chromosomal microarray analysis.
Thus, in most cases, Edwards syndrome is the result of random errors in cell division and has little to do with any environmental factors or human condition.
Literature
- Abramsky L, Chapple J. Room for improvement? Detecting autosomal trisomies without serum screening. Public Health 1993;107:349-354.
- Baty BJ, Blackburn BL, Carey JC. Natural history of trisomy 18 and trisomy 13. I. Growth, physical assessment, medical histories, survival, and recurrence risk. Am J Med Genet 1994;49:175-188.
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic urban cohort. Environmental Health Perspectives 2003;111:1:79-84.
- Botto LD, Mulinare J, Yang Q, Liu Y, Erickson JD. Autosomal trisomy and maternal use of multivitamin supplements. American Journal of Medical Genetics 2004:125A:113-116.
- Buyse ML, editor-in-chief. Birth Defect Encyclopedia. Cambridge, Massachusetts: Blackwell Scientific Publications, 1990.
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, Devine O, Petrini J, Ramadhani TA, Hobbs CA, Kirby RS. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Res A Clin Mol Teratol. 2006 Nov;76(11):747-56.
- Canick JA, Saller DN. Maternal serum screening for aneuploidy and open fetal defects. Obstet Gynecol Clin North Am 1993;20:443-454.
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. The Lancet Oncology 2004;5:283-291.
- Carothers AD, Boyd E, Lowther G, Ellis PM, Couzin DA, Faed MJ, Robb A. Trends in prenatal diagnosis of Down syndrome and other autosomal trisomies in Scotland 1990 and 1994, with associated cytogenetic and epidemiological findings. Genet Epidemiol 1999;16:179-190.
- Cedergren MI, Selbing AJ, Lofman O, Kallen BAJ. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environmental Research Section A 2002;89:124-130.
- Chen M, Yeh GP, Shih JC, Wang BT. Trisomy 13 mosiacism: study of serial cytogenic changes in a case from early pregnancy to infancy. Prenatal Diagnosis 2004;24:137-143.
- Cordier S, Chevrier C, Robert-Gnansia E, Lorente C, Brula P, Hours M. Risk of congenital anomalies in the vicinity of municipal solid waste incinerators. Occup Environ Med 2004;61:8-15.
- Doran TA, Cadesky K, Wong PY, Mastrogiacomo C, Capella T. Maternal serum alpha-fetoprotein and fetal autosomal trisomies. Am J Obstet Gynecol 1986;154:277-281.
- Forrester MB, Merz RD. Trisomies 13 and 18: Prenatal diagnosis and epidemiologic studies in Hawaii, 1986-1997. Genet Test 1999;3:335-340.
- Forrester MB, Merz RD, Yoon PW. Impact of prenatal diagnosis and elective termination on the prevalence of selected birth defects in Hawaii. Am J Epidemiol 1998;148:1206-1211.
- Fried PA. The consequences of marijuana use during pregnancy: a review of the human literature, in Women and Cannabis: Medicine, Science, and Sociology, The Haworth Integrative Healing Press, 2002.
- Gessner B.D. Reasons for trisomy 13 and 18 births despite the availability of prenatal diagnosis and pregnancy termination. Early Human Development 2003; 73:53-60.
- Goldstein H, Nielsen KG. Rates and survival of individuals with trisomy 13 and 18. Clin Genet 1988;34:366-372.
- Greenberg F, Schmidt D, Darnule AT, Weyland BR, Rose Esmie, Alpert E. Maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, and unconjugated estriol levels in midtrimester trisomy 18 pregnancies. Am J Obstet Gynecol 1992;166:1388-1392.
- Harrison R.M. Hazardous waste landfill sites and congenital anomalies. Occup Environ Med 2003; 60:79-80.
- Hassold TJ, Burrage LC, Chan ER, Judis LM, Schwartz S, James SJ, Jacobs PA, Thomas NS. Maternal folate polymorphisms and the etiology of human nondisjunction. American Journal of Human Genetics; 2001:69:434-439.
- Hook EB, Topol BB, Cross PK. The natural history of cytogenetically abnormal fetuses detected at midtrimester amniocentesis that are not terminated electively: New data and estimates of the excess and relative risk of late fetal death associated with 47,+21 and other abnormal karyotypes. Am J Hum Genet 1989;45:855-861.
- Huether CA, Martin LM, Stoppelman SM, D'Souza S, Bishop JK, Torfs CP, Lorey F, May KM, Hanna JS, Baird PA, Kelly JC. Sex ratios in fetuses and liveborn infants with autosomal aneuploidy. Am J Med Genet 1996;63:492-500.
- Jeng W, Wong AW, Ting-A-Kee R, Wells PG Methanmphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radical Biology and Medicine 2005; 39:317-326.
- Kallen B. Use of antihistamine drugs in early pregnancy and delivery outcome. Journal of Maternal-Fetal and Neonatal Medicine 2002;11:146-152.
- Kline J, Kinney A, Levin B, Warburton D. Trisomic pregnancy and earlier age at menopause. American Journal of Human Genetics 2000;67:395-404.
- Kupke KG, Muller U. Parental origin of the extra chromosome in trisomy 18. Am J Hum Genet 1989;45:599-605.
- Munne S, Sandilinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenatal Diagnosis 2004; 24: 638-643.
- Naguib KK, Al‑Awadi SA, Moussa MA, Bastaki L, Gouda S, Redha MA, Mustafa F, Tayel SM, Abulhassan SA, Murthy DS. Trisomy 18 in Kuwait. Int J Epidemiol 1999;28:711-716.
- Nothen MM, Eggermann T, Erdmann J, Eiben B, Hofmann D, Propping P, Schwanitz G. Retrospective study of the parental origin of the extra chromosome in trisomy 18 (Edwards syndrome). Hum Genet 1993;92:347-349.
- Palermo GD, Neri QV, Hariprashad JJ, Davis OK, Veeck LL, Rosenwaks Z. ICSI and its outcome. Seminars in Reproductive Medicine 2000; 18:2: 161-169.
- Parker MJ, Budd JLS, Draper ES, Young ID. Trisomy 13 and trisomy 18 in a defined population: epidemiological, genetic, and prenatal observations. Prenatal Diagnosis 2003;23:856-860.
- Park-Wyllie L, Mazzotta P, Pastuszak A, Moretti ME, Beique L, Hunnisett L, Friesen MH, Jacobson S, Kasapinovic S, Chang D, Diav-Citrin O, Chitayat D, Nulman I, Einarson TR, Koren G. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000;62:385-392.
- Pradat P. Is trisomy 18 increasing in Sweden? An analysis of the syndrome during a ten-year period and a comparison with a French registry. Hereditas 1991;114:97-102.
- Ramesh KH, Verma RS. Parental origin of the extra chromosome 18 in Edwards syndrome. Ann Genet 1996;39:110-112.
- Rasmussen SA, Wong LYC, Yang Q, May KM, Friedman JM. Population based analysis of mortality in trisomy 13 and trisomy 18. Pediatrics 2003;111:777-784.
- Schreinemachers DM, Cross PK, Hook EB. Rates of 47,+21, 47,+18, 47,+13 and other chromosome abnormalities in about 20,000 prenatal studies compared with estimated rates in live births. Hum Genet 1982;61:318-324.
- Sorensen HT, Czeizel AE, Rockenbauer M, Steffensen FH, Olsen J. The risk of limb deficiencies and other congenital abnormalities in children exposed to calcium channel blockers. Acta Obst Gynecol Scand 2001;80:397-401.
- Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Antenatal evaluation and management of ultrasonically detected fetal anomalies. Obstet Gynecol 1987;69:640-660.
- Wennborg H, Magnusson LL, Bonde JP, Olsen J. Congenital malformations related to maternal exposure to specific agents in biomedical research laboratories. JOEM 2005; 47:1:11-19.
- Vrijheid M, Dolk H, Stone D, Abramsky L, Alberman E, Scott JES. Socioeconomic inequalities in the risk of congenital anomaly. Arch Dis Child 2000; 82:349-352.
- Ya-gang X, Robinson WP, Spiegel R, Binkert F, Ruefenacht U, Schinzel AA. Parental origin of the supernumerary chromosome in trisomy 18. Clin Genet 1993;44:57-61.
Frequency
In 60 cases of mutations out of 100, children with the syndrome in question die inside the mother’s abdomen, because the defects are incompatible with life. But the survival rate of children with Edwards syndrome is quite high (slightly lower than that of fetuses with trisomy 21). For every 3-8 thousand babies, one is born with the diagnosis in question.
Doctors say the disease is three times more common among female babies than among boys. There is a high risk of giving birth to a child with these abnormalities in women giving birth who are over 30 years old. During the first 12 months of life, about 90 out of 100 children with this diagnosis die. Boys live on average from 2 to 3 months, and girls about 10 months. The chances that a child with Edwards syndrome will survive into adulthood are slim. Complications of developmental defects become causes of death in children:
- intestinal obstruction
- cardiovascular failure
- pneumonia
- suffocation
Prevention
Speaking about the prevention of the disease, it should be noted that the risk of its development in the unborn child always exists. But it still increases in parents after 40 years of age.
Therefore, it is very important for a pregnant woman to follow all the instructions of the gynecologist observing her and undergo an antenatal screening procedure for the timely detection of pathologies.
If one of the spouses has an unfavorable family history, then at the pregnancy planning stage you should definitely visit a genetic specialist and follow all his advice.
Symptoms
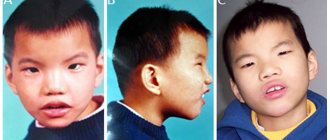
Manifestations of the disease are divided into several groups. The first includes those that characterize the appearance of a sick person:
- body weight at birth is approximately 2 kg 100 grams or 2 kg 200 grams
- abnormally developed lower or upper jaw
- the head is small in relation to the whole body
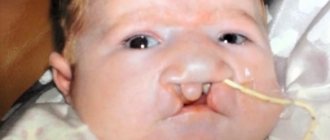
- cleft lip and/or hard palate
- malocclusion and irregular face shape of the child
- rocking foot
- clubfoot from birth
- webbed toes or complete fusion of the toes
- ears set low
- the fingers of the hand are clenched, their placement in the fist is uneven
- the mouth gap is smaller than it should be
The second group of symptoms of the disease concerns the neuropsychic sphere, motor skills and organ function of the sick child:
- umbilical or inguinal hernia
- congenital heart defects, including patent ductus arteriosus, ventricular septal defect, etc.
- smoothing or atrophy of cerebral convolutions
- underdevelopment of the cerebellum, corpus callosum
- mental retardation
- delayed neuropsychic development of a child
- violation of intestinal location
- Meckel's diverticulum
- atresia of the esophagus or anus
- disturbance of the swallowing and sucking reflex
- GERD
- duplication of ureters
- horseshoe-shaped or segmented bud
- underdevelopment of the ovaries in girls
- hypertrophied clitoris in female infants
- hypospadias in male infants
- cryptorchidism in sick boys
- amyotrophy
- scoliosis
- strabismus
Diet
Diet when planning pregnancy
- Efficacy: no data
- Timing: 3-5 months before planned pregnancy
- Cost of products: 1800-2000 rubles. in Week
Children with this pathology require special nutrition. In severe cases, in the absence of swallowing and sucking reflexes, feeding is carried out through a tube.
Expectant mothers before and after conception need to eat healthy, without consuming harmful foods or alcohol.
Diagnostics
You can most often find out about genetic pathologies while a woman is still carrying a child. This also applies to trisomies. Pregnancy screening is carried out from the 11th to the 13th week. The woman takes blood tests (biochemistry) and an ultrasound is performed. Diagnosis also involves determining the karotype of the embryo if the woman is at risk (complicated family history, infectious diseases in the first trimester, etc.).
During the first semester screening, the amount of human chorionic hormone and plasma protein A associated with pregnancy is determined in the blood. Then they take into account the age of the pregnant woman to find out what her risk is of having a child with trisomy 18.
If a woman is classified as a risk group, a fetal biopsy is performed a little later in order to know for sure whether the child will be born with abnormalities or healthy. From 8 to 12 weeks, chorionic villus sampling is taken. From the 14th to the 18th week, the waters surrounding the fetus are studied. After the 20th week, cordocentesis can be done. The procedure involves taking blood from the umbilical cord (ultrasound is used in the process to control the collection of material).
The number of chromosomes is detected in the material. The CP-PCR method helps with this. If the pregnant woman fails genetic screening for late gestation, a preliminary diagnosis of the genetic mutation is made using ultrasound. In the second and third trimester, there are signs that indicate that the child is likely to be born with trisomy:
- cleft lip
- low-set fetal ears
- microcephaly
- cleft palate
- defects of the musculoskeletal system
- malformations of the genitourinary system
- heart and vascular defects
How to take the Prenetix test?
To take the Prenetix test, you need to apply for the test and donate blood. Blood sampling for testing can be done in any region of the country - in specialized medical institutions or laboratories that have an agreement with CGRM Genetico. Next, the material will be sent for analysis to the Moscow Genetico laboratory. After 12 days you will be able to get the result.
For more detailed information and to sign up for analysis, contact our specialists at the indicated telephone numbers or through the online form on the website.
Diagnosis after birth
Edwards syndrome in newborns is detected by the following signs:
- low birth weight
- microcephaly
- cleft palate or cleft lip
- transverse palmar groove
- undeveloped distal flexion fold on the fingers
- distal location of the axial triradius and increase in ridge count on the palm
- arches on the fingertips
But these signs do not yet indicate that the child has Edwards syndrome. The diagnosis needs confirmation. To do this, the above-mentioned CP-polyplasma chain reaction method is used to determine caropine in a newborn. Ultrasound shows choroid plexus cysts in an infant.
Risk factors
The main risk factors are age (especially significant for Down syndrome), as well as exposure to radiation and certain heavy metals. It should be borne in mind that even without risk factors, the fetus can have pathology.
As can be seen from the graph, the dependence of the risk on age is most significant for Down syndrome, and less significant for the other two trisomies.
Diagnosis by ultrasound before birth
The following indirect signs of the disease are detected, starting from the twelfth week of gestation:
- 1 rather than 2 umbilical arteries
- The nasal bones are not visualized on ultrasound
- abdominal hernia
- low heart rate
- choroid plexus cysts
Cysts are not dangerous for the baby; they are eliminated by the 26th week of gestation. But such cysts indicate that the child has a genetic developmental abnormality. This may be the syndrome discussed in this article. Cysts are found in a third of patients with this diagnosis. If the doctor sees cysts on an ultrasound, then the next stage of prenatal diagnosis is a consultation with a geneticist.
Classification
Complete trisomy - if a whole additional chromosome appeared at the stage of one cell, then the set of chromosomes is disrupted in each cell of the fetus. With this form, the child has numerous developmental defects that are incompatible with life. This is the most severe version of the disease.
Partial trisomy is diagnosed if an extra chromosome does not appear at the earliest stages of fetal development. In this case, a violation of the genetic makeup is not observed in all cells, and some of them will remain healthy.
Treatment of Edwards syndrome
The goal of therapy: to correct developmental defects that are life-threatening to the child. But it is worth remembering that the child may have serious disabilities and is unlikely to survive beyond 12 months of age. If pneumonia is detected, the baby is given anti-inflammatory drugs and antibiotics. If it is discovered that the baby does not have a sucking and swallowing reflex, then he is fed using a tube. If anal or intestinal atresia is detected in a patient, the passage of food must be restored.
If the doctor sees that the course of Edwards syndrome is favorable, then surgery must be performed to eliminate the umbilical hernia, inguinal hernia, heart defects, and cleft palate. Some symptoms can be relieved by taking medications. For example, if a baby is constipated, he or she will need certain laxative medications. When gases accumulate in the intestines, drugs from a number of “defoamers” are prescribed.
Children who have been diagnosed with the trisomy in question are at risk for the following diseases:
- genitourinary infections
- sinusitis and sinusitis
- arterial hypertension
- apnea
- pulmonary hypertension
- pneumonia
- kidney cancer
- otitis media
It is important to detect these diseases in a baby in time and treat them correctly. The prognosis in most cases of the disease is unfavorable. As already noted, a child has an extremely low chance of surviving to puberty. If the child survives, he will need constant care and supervision because his brain will not be sufficiently developed. Some patients can eat without the help of others, smile and acquire minimal skills.
Pathogenesis
Trisomy 18 is a genetic pathology. Each human cell contains 46 chromosomes - these are normal values. During fertilization, 23 chromosomes of female and male germ cells combine and give a total of exactly this number of chromosomes. Sometimes, for unknown reasons, genetic mutations occur, and as a result, an extra 47th chromosome appears, additional to the 18th pair. An extra chromosome appears in gametes as a result of chromosome nondisjunction during meiotic division. In most cases - 95% - it is the extra chromosome that causes the development of Edwards disease. But sometimes - in about 2% of cases - the sum of chromosomes remains normal, but chromosome 18 is abnormally lengthened, which leads to the development of congenital pathology. In another 3% of cases, mosaic trisomy is observed, in which an additional chromosome is not present in all cells of the body, but only in some part of it. All three forms of the syndrome follow the same type. But still, a more severe course is observed in the first form of the disease.