What are basophils, functions
In the KLA, the indicator is present in the leukogram. Basophils are blood cells from the group of granulocytes. Produced by the bone marrow, they are a type of white blood cell. Viability is maintained from seven to twelve days.
Establishing the level of basophils helps to identify pathological inflammation or the presence of allergic reactions. The resulting focus of inflammation attracts basophils as part of other leukocytes. They are then transformed into heparinocytes, which are involved in the production of histamine, as well as serotonin and heparin. Histamine is designed to combat hypersensitivity, and heparin reduces the ability of blood to clot. Serotonin expands the vascular lumen and increases the ability of the vascular membranes to absorb and release substances.
The cells contain prostaglandins, the purpose of which is to prevent the action of the irritant and neutralize it. At this time, a person feels hyperthermia, becomes weak, and has a fever.
The main purpose of basophils is to take part in immediate and delayed hypersensitivity reactions. The cells are the first to reach the source of inflammation and signal the presence of foreign agents. If the inflammation process lasts longer than three days, more and more basophils are produced in the body.
Main functions:
- trigger the blood clotting reaction;
- participate in the creation of new blood vessels;
- participate in triggering allergic reactions;
- help increase capillary permeability;
- protect the body from infections and parasitic infestations.
A normal indicator is the presence of no more than 1% basophils in the blood. A reduced amount (basopenia) and an increased amount (basophilia) are grounds to suspect an inflammatory process or blood pathology.
IgE, mast cells, basophils and eosinophils
IgE, mast cells, basophils and eosinophils are important elements of allergic inflammation. Allergen-specific IgE, synthesized in response to allergens in the environment and in susceptible individuals, becomes fixed to high-affinity receptors on cell membranes, especially mast cells and basophils. If these receptor-bound IgE molecules aggregate upon repeated exposure to a specific allergen, these mast cells and basophils produce mediators that lead to an allergic response. The main cells attracted to mediator release sites are eosinophils.
IgE
Reagin antibody IgE (immunoglobulin E) has an approximate molecular weight of 190 kDa, does not have transplacental transfer and, unlike other immunoglobulins, does not activate complement through the classical pathway. IgE is heat labile and will not be sensitized after being heated to 56°C for several hours. IgE is mainly known for its ability to bind with high affinity to its specific receptor (R) FcϵRI, found in its full form (α 2 ) on the membranes of mast cells and basophils. Serum IgE concentration is the lowest of the five human immunoglobulin isotypes (0-0.0001 g/L, representing 0.004% of total serum immunoglobulin) and is highly age dependent. Serum IgE concentrations are low in umbilical cord serum (<2 kU/L, <4.8 mg/L) and increase with age until the individual reaches 10–15 years of age. . People with an allergic predisposition experience an earlier and steeper rise. Total serum IgE levels decline from the second to eighth decades of life. According to experts, about 50% of the body's total IgE is found inside the blood vessels. IgE has a half-life of 1 to 5 days in peripheral blood.
B cells initially produce IgM antibodies, but after appropriate stimuli they change the isotype of the antibody produced, maintaining antigen specificity by sharing the same variable (VDJ) region. This “isotype switching” is effective in the sense that it allows one B cell clone to produce antibodies with the same specificity but different effector functions found in the constant portion of the heavy chain. This process consists of splicing and rejoining genomic DNA to map VDJ elements to C-region exons that encode, in the case of IgE, the ϵ-strand that determines the IgE isotype. IgE synthesis requires two types of signals. Signal 1 is provided by the cytokines interleukin (IL)-4 and IL-13, which activate transcription at a specific immunoglobulin locus. The second signal is provided by CD40 ligation on B cells, which in turn activates DNA switch recombination. Both signals are represented by B cells and T cells.
The process begins when the allergen binds to allergen-specific IgM on a B cell, which then processes the allergen. When the B cell then presents fragments of this allergen in the context of MHC class II molecules in the T cell receptor-CD3 complex on a T H 2 cell, the T cell rapidly expresses IL-4 ligand and CD40 (CD40L, CD154), CD40L includes CD40, expressed in the B cell. This interaction results in the expression of B7 on B cells, which binds to CD28 on the T cell, resulting in upregulation of T cell-derived IL-4.
Human mast cells and basophils have been reported to secrete IL-4, IL-13, or both, and also express some CD40L. Thus, these observations suggest that these cells may interact with B cells to provide signals for IgE synthesis or amplification. Since the production of IL-4 or IL-13 by basophils and mast cells depends on the aggregation of Fc-RI through antigen-specific IgE, this mechanism is more attractive for amplification of IgE synthesis. This mechanism does not appear to be allergen specific, but rather produces a polyclonal response. This hypothesis is consistent with the observation that the IgE response in hyper-IgE states is polyclonal.
There are two different IgE receptors: the low-affinity IgE receptor (FcϵRII; CD23), present on B cells, and the high-affinity IgE receptor (FcϵRI). Fc-RI on mast cells and basophils is a tetramer (α2), whereas it is expressed in a trimeric form (2) on antigen-presenting cells such as monocytes, Langerhans cells, and peripheral blood dendritic cells. Human basophil human Fc-RI expression density correlates with serum IgE levels because IgE binding to Fc-RI stabilizes the receptor on the cell surface. Fc-RI-IgE interactions may also promote mast cell survival.
Total IgE levels are affected by age, genetic predisposition, ethnicity, immune status, season and certain diseases. Elevated levels of IgE are found in parasitic diseases such as schistosomiasis and hookworm; infections such as allergic bronchopulmonary aspergillosis and Epstein-Barr mononucleosis virus; skin diseases such as bullous pemphigoid; tumor diseases such as Hodgkin's disease and IgE myeloma; immunodeficiency diseases such as Wiskott-Aldrich syndrome, hyperimmunoglobulinemia E syndrome, thymic hypoplasia (DiGeorge syndrome) and cellular immunodeficiency with immunoglobulins (Nezelof syndrome); and a number of other diseases such as nephrotic syndrome, cystic fibrosis, Kawasaki disease and polyarteritis nodosa. Taken together, such information dictates that measurement of total serum IgE levels is of limited value as a screening test for allergic disease.
Basophils
Basophils are granulocytes that are believed to represent a separate lineage from mast cells, despite the fact that the two cell types share many features such as high-affinity IgE receptor (FcϵRI) expression, metachromatic staining, TH 2 cytokine expression and release histamine. Basophils make up less than 1% of peripheral blood leukocytes, making them one of the least abundant cell lineages in peripheral blood. The number of basophils in peripheral blood is slightly increased (approximately 2 times) in allergic asthma. Basophils have a segmented nucleus with highly condensed chromatin and are usually identified by metachromatic staining with basic dyes such as toluidine blue. Recently, two monospecific antibodies to basophil granules, BB-1 and 2D7, have been developed that allow the unambiguous identification of basophils in tissues and significantly expand our understanding of the role of basophils in allergic diseases and asthma. Basophils express various cytokine receptors (IL-3R, IL-5R, GM-CSFR), chemokine receptors (CCR2, CCR3), complement receptors (CD11b, CD11c, CD35, CD88), prostaglandin receptors (CRTH2) and immunoglobulin Fc receptors ( FcϵRI, FcγRII).
Basophils develop from CD34+ pluripotent stem cells, differentiate and mature in the bone marrow, and then circulate in the periphery. IL-3 is the dominant cytokine that drives basophil differentiation and is sufficient for the differentiation of stem cells into basophils. The general consensus is that basophils are a separate cell lineage from mast cells and are distinct from the common basophil-eosinophil precursor; This belief is supported by the origin of mixed colonies of basophils and eosinophils from individual progenitor cells.
As with mast cells, basophils express a complete and functional Fc-RI receptor (αβ 2 ), the cross-linking of which leads to basophil activation, exocytosis of granules. and release of the intermediary. C3a and C5a can also activate basophils through the complement receptors C3aR and C5aR, respectively. Activation through any of these receptors results in histamine release, eicosanoid synthesis, and IL-4 and IL-13 gene expression. Priming is the ability of molecules that cannot maximally activate basophils on their own to enhance Fc-RI-mediated activation. Mediators with such priming activity include CC chemokines (eotaxins, monocyte chemoattractant protein 3, monocyte chemoattractant protein 4, RANTES), N-formyl-methionyl-leucyl-phenylalanine, IL-3, IL-5, GM-CSF, and histamine releaser. factor. The presence of such mediators at sites of allergen exposure can reduce the threshold for the development of allergic inflammation.
Basophils produce many mediators similar to mast cells, such as histamine, leukotrienes, IL-4 and IL-13. Conversely, the mast cell mediators PGD 2 and IL-5 are not produced by basophils. Basophils mainly generate LTC 4 from newly synthesized eicosanoid mediators. In addition to histamine, basophil granules contain a number of other preformed mediators such as chondroitin sulfate, basic basic protein, and Charcot-Leyden crystalline protein. Typically, basophils contain only small amounts of tryptase; however, there appears to be a lot of variation between individuals regarding basophil tryptase expression. In addition to their role in direct hypersensitivity, basophils may contribute to allergic inflammation through a number of non-classical mechanisms. Basophilic expression of IL-4 and CD40L induces IgE B cell switching in vitro and may contain an alternative mechanism promoting IgE class switching. Alternatively, rapid and abundant expression of IL-4 by basophils has been proposed as a source of IL-4 that may further stimulate TH 2 cell differentiation.
The physiological role of basophils is unknown, although, like other leukocytes, they appear to have a role in host defense. Basophils have long been thought to play a role in tick rejection and are a prominent component of the inflammatory response to many parasites. This proposed role in host defense against the parasite is supported by the recent discovery of functional homologs of the parasite histamine releasing factor in the translationally controlled family of tumor proteins. Although basophils have many characteristics that suggest they contribute to allergic inflammation, the precise role of basophils in the pathogenesis of asthma is unclear. Following allergen challenge, basophils are the predominant IL-4-expressing cell type in human asthmatic airway peripheral blood mononuclear cells.
Eosinophils
Eosinophils are granulocytes that were first described to stain with acid aniline dyes such as eosin. Although these cells are rarely found in the peripheral blood of healthy individuals, eosinophilia in the blood and tissues is a hallmark of helminth infections, allergies, and asthma. Due to the large body of evidence supporting a critical role in the pathogenesis of asthma, the eosinophil has emerged as a major therapeutic target for immunologic therapy of asthma.
Eosinophils typically have a binucleated nucleus with highly condensed chromatin and cytoplasm containing two main types of granules: specific and primary granules. Specific granules have a characteristic ultrastructural appearance consisting of an electron-dense crystalloid core. These granules contain many cationic proteins that give eosinophils their unique staining properties. Primary granules are similar to those found in other granulocyte lineages and are found early in eosinophil development. Eosinophils also contain lipid bodies that play a role in the generation of eicosanoid mediators.
Since there is no cell surface marker specific for eosinophils, their binding to eosin-like dyes remains the most common detection method. The development of monoclonal antibodies to various eosinophil granule proteins has provided an additional means of immunochemical identification of these cells. Eosinophils express a wide range of cell surface molecules, including cytokine receptors (IL-3R, IL-5R and GM-CSFR), chemokine receptors (CCR1 and CCR3), FcγRII (CD32), FcαRI (secretory IgA) complement receptors (C3aR, C5aR, CD88 and CD35), adhesion molecules (very late antigen [VLA]-4 and α4β7 integrin), CD9 and CD69. CD69 is a marker of eosinophil activation and is upregulated on eosinophils isolated from sites of allergic inflammation. Eosinophilic FcϵRI expression is minimal and has unclear functional significance.
Eosinophils develop and mature in the bone marrow from CD34+ progenitor cells and are released into the peripheral blood as mature cells. IL-5, the major eosinophil active cytokine, has profound beneficial effects on the differentiation and proliferation of eosinophil precursors in the bone marrow. Thus, IL-5 is produced peripherally at sites of allergic inflammation, or helminth infection acts distally on the bone marrow. In addition, allergenic stimulation or experimental administration of eotaxin causes the release of mature eosinophils as well as eosinophil precursors in the bone marrow.
After release from the bone marrow, eosinophils circulate in the peripheral blood and then enter tissues with a half-life in the peripheral blood of 8 to 18 hours. Although eosinophils are best known as peripheral blood white blood cells, the vast majority of eosinophils are found in the intestine and lungs. Eosinophils are also the main source of cysteinyl leukotriene LTC 4 and its active metabolites LTD 4 and LTE 4. Eosinophils, along with mast cells and basophils, are the main LTC 4 synthesis-producing cells in the mucous membrane of bronchial asthma. Eosinophils are capable of producing a number of cytokines, including IL-1, transforming growth factor β, IL-3, IL-4, IL-5, IL-8, and TNF-α. However, eosinophils typically produce fewer cytokines than other inflammatory cells such as T cells. As such, the relative contribution of eosinophil cytokine production to the generation of allergic inflammation remains to be determined.
The number of eosinophils in the peripheral blood increases in allergic diseases, asthma and helminthiasis, and tissue eosinophilia is often found at sites of inflammation associated with these diseases. Despite their in vitro activity against parasites, in vivo studies in IL-5 knockout mice generally do not support a significant role for eosinophils in clearing parasitic infections. In allergic diseases and asthma, eosinophils play a proinflammatory role, in which eosinophilic mediators such as MBP are thought to cause mucosal inflammation and consequent bronchial hypersensitivity. Corticosteroids strongly reduce the number of eosinophils in peripheral blood and tissues, further supporting the central role of eosinophils in the pathogenesis of asthma. Because eosinophils are considered the final effector cell of asthma, a number of investigational treatments have targeted eosinophils. Recently, a phase II study of anti-IL-5 in human asthma demonstrated no improvement in either airflow or late-phase allergic reactions, despite a 90% reduction in peripheral blood eosinophils. A subsequent anti-IL-5 study demonstrated a similar 90% reduction in peripheral blood eosinophils, but only a 55% reduction in bronchial mucosal eosinophils. These data demonstrate that anti-IL-5 alone may not be sufficient to reverse pulmonary eosinophilia and that additional anti-eosinophil strategies need to be explored to determine their therapeutic potential in the treatment of asthma.
When is it necessary to determine basophils in the blood?
A blood test to determine the level of basophils is carried out:
- during routine medical examinations;
- during examination before hospitalization for surgery;
- for the purpose of diagnosing infectious diseases, inflammatory phenomena, blood pathologies;
- to monitor the correctness of treatment.
The results are interpreted within the limits of the leukocyte formula; blood is not taken separately for basophils in laboratories.
Based on the level of this type of leukocyte, the doctor receives information about the presence/absence of an inflammatory process, the occurrence of allergic reactions, and may suspect a malignant blood disease. The result must be interpreted by a doctor: hematologist, infectious disease specialist, internist or pediatrician.
Reasons for low level
A condition in which basophils are low is called basopenia. At the same time, their level drops below 0.01X10⁹ per liter. The reasons for low basophils may be the following:
- Acute infectious diseases.
- Acute pneumonia.
- Severe stress.
- Itsenko-Cushing syndrome.
- Hyperfunction of the thyroid gland.
- Pregnancy.
- Taking certain medications.
- Consequences of chemotherapy.
- The relative indicator is low during the recovery period for infectious diseases.
Any deviations in basophil levels should not be left without medical attention.
If estrogens are able to enhance the process of basophil formation, then glucocorticosteroids and another female hormone, progesterone, on the contrary, reduce their level. You can read about the reasons for the increase in basophils in this article.
Normal indicators
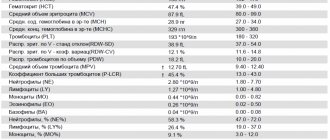
The blood test form contains two indicators: relative, which shows the proportion of cells in the total number of leukocytes (BA%) and absolute, showing the number of basophils (BA#).
Each laboratory indicates its own normal ranges on the form, since indicators differ among clinics due to the specific equipment used for laboratory tests.
The number of basophils does not depend on gender, age, or time of day. Normally, the relative indicator is: 0-1%, absolute: 0-0.09*109/l. The absolute value gives a more complete picture of health. Sometimes it may be necessary to manually determine cell counts under a microscope.
The interpretation of the tests is carried out by the doctor as a whole, so based on only one indicator it is impossible to establish an accurate diagnosis.
Why is a leukocyte blood formula needed?
The leukocyte formula is the percentage of leukocytes in the blood serum (eosinophils, neutrophils, lymphocytes, basophils, monocytes).
This analysis allows you to determine the current state of the immune system, identify inflammatory processes in the patient’s body and determine the etiology of allergies. It is known that leukocytes protect the human body from dangerous microorganisms. One of the main tasks of leukocytes is the destruction of foreign particles. If an inflammatory process occurs in the patient’s body, it is immediately reflected in the leukocyte count.
Complete blood count (CBC/Diff - 5 fractions of leukocytes) - capillary blood in Moscow from day 1
from 290 ₽
Sign up
Clinical blood test (CBC/Diff - 5 leukocyte fractions) + ESR in Moscow from day 1
from 294 ₽
Sign up
Complete blood count (CBC/Diff - 5 fractions of leukocytes) in Moscow from 1 weekday
from 290 ₽
Sign up
When leukocyte counts change in the blood, it is necessary to determine in which direction the deviation occurs. This study will help you quickly find the problem and make a diagnosis. However, it should be taken into account that changes in blood parameters without in-depth diagnostics are not a characteristic and final sign for making a diagnosis.
Why basophils may be elevated
Basophilia is diagnosed when the number of cells exceeds 0.2*109 /l. The use of drugs with estrogen hormones, as well as the use of thyreostatic drugs, can distort the picture. An increase in the rate is observed at the beginning of the menstrual cycle in women.
Basophils are increased:
- for chronic diseases of the gastrointestinal tract: inflammation of the gastric mucosa, enterocolitis, ulcerative inflammation of the large intestine, ulcerative lesions of the stomach and duodenum;
- at the stage of weakening of acute symptoms of an infectious disease;
- for blood diseases: acute form of leukemia, chronic myeloid leukemia, anemia, polycythemia, hemophilia, lymphogranulomatosis;
- for allergies and diabetes;
- with a decrease in the hormonal activity of the thyroid gland - hypothyroidism;
- oncology of the lymphatic system, blood cancer, lung cancer;
- due to prolonged exposure to low doses of radiation.
The main reasons for the increase in the indicator are a violation of immune defense and active invasion of the pathogen. In an adult, basophils may be elevated after removal of the spleen.
A child's concentration may increase:
- due to taking medications that cause allergies;
- due to a hidden inflammatory process;
- due to a recent vaccination or mosquito bite that gives rise to allergies;
- due to the introduction of foreign proteins.
To exclude pathology, a repeat analysis is recommended a week after the cessation of the above reasons. If the indicators are high again, a comprehensive examination is prescribed to identify the exact cause.
Leukocyte formula:
| Index | × 10x9/l | ratio, % | |
| Neutrophils | segmented | 2,1–5,4 | 43–71 |
| stab | 0,4–0,3 | 1–5 | |
| Basophils | up to 0.063 | up to 1 | |
| Eosinophils | 0,02–0,3 | 0,5–5 | |
| Lymphocytes | 1,1–3,1 | 17–38 | |
| Monocytes | 0,08–0,5 | 3–12 | |
How to reduce the number of basophils
As we said above, basophilia is not an independent disease, but can only be considered as a marker of a pathological process, indicating certain changes in the body, so it is not worth fighting this symptom. However, this sign cannot be ignored and additional examination is necessary to identify the causes that led to this condition and act on them.
If the cause of basophilia was anemia, iron supplements, vitamins B12 and folic acid are prescribed. Antihistamines are used to treat allergies. If physiological basophilia occurs, correction is usually not required.
After a correct diagnosis and adequate therapy, when the cause of the pathological process is eliminated, the blood test returns to normal.
Return to articles
Leukocytes and their significance
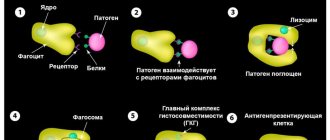
White blood cells - the main group to which basophils belong - are otherwise called leukocytes, and their most important function is considered to fight threats to the body. Leukocytes are divided into groups according to the structure of the nucleus and the presence of granules with active substances in the cytoplasm; the latter phenomenon is also called graininess. The structure also determines the variation in functionality: many leukocytes are, to one degree or another, capable of performing the same functions (for example, capturing foreign agents), but each type of cell is usually busy with its own business and is adapted for a specific task.
An increase in the number of leukocytes indicates the body’s readiness to resist harmful effects, and basophils cannot be an exception to the rule. There are two concepts that imply an increase in the number of these cells - basophilia and basophilocytosis.
What does a shift in the leukocyte formula to the left and to the right mean?
Neutrophils form the body's antibacterial and antifungal defenses, and when a certain microbe enters the body, the number of neutrophils increases. In this case, not only their total number changes, but also the number of individual forms of these cells.
Chain of neutrophils arranged according to maturation:
Young - rod-nuclear - segmented.
A shift of the formula to the left is an increase in the number of young cells, and a shift to the right is an increase in the number of old cells.
Reasons for the shift in leukocyte formula
In medical practice, a shift to the left occurs more often. This is affected by the presence of an acute bacterial or fungal infection in the body. The bone marrow is mobilized to protect the body and begins to intensively produce neutrophils. They begin to fight the infection and die in the process. Young cells are produced to replace mature cells. At a certain point, the number of young cells exceeds the number of mature ones.
An increase in segmented neutrophils leads to a shift in the leukocyte count to the right. This occurs not only against the background of an increase, but even more often with a decrease in the number of leukocytes. This is facilitated by long-term chronic infections, in which bone marrow reserves are depleted and young cell forms cease to form. Poisoning, radiation, chemotherapy and radiotherapy, which also suppress the bone marrow, can cause the correct shift.
To prevent changes in the number of leukocytes and changes in the leukocyte formula, you need to monitor your health. To maintain your immune system, you should exercise, maintain a work-rest schedule, eat right, and avoid stressful situations. Also, do not forget about undergoing a routine examination with a therapist.
Table 1
| Index | Designation | In men | Among women |
| Red blood cells (x 1012/l) | R.B.C. | 4-5,1 | 3,7-4,7 |
| Average erythrocyte volume (fl or µm3) | MCV | 80-94 | 81-99 |
| Erythrocyte sedimentation rate (mm/h) | ESR | 2-15 | 2-10 |
| Anisocytosis of erythrocytes (%) | RDW | 11,5-14,5 | 11,5-14,5 |
| Hemoglobin (g/l) | HGB | 130-160 | 120-140 |
| Average hemoglobin level in erythrocyte (pg) | MCH | 27-31 | 27-31 |
| Average erythrocyte hemoglobin concentration (%) | MCHC | 33-37 | 33-37 |
| Color index | CPU | 0,9-1,1 | 0,9-1,1 |
| Hematocrit (%) | HCT | 40-48 | 36-42 |
| Platelets (x 109/l) | PLT | 180-320 | 180-320 |
| Average platelet volume (fl or µm3) | MPV | 7-11 | 7-11 |
| Reticulocytes (%) | RET | 0,5-1,2 | 0,5-1,2 |
| Leukocytes (x 109/l) | WBC | 4-9 | 4-9 |
Neutrophils
Neutrophils come from red bone marrow, they are formed from a single stem cell, which is the ancestor of all blood cells. However, stem cells do not immediately turn into neutrophils. Between these two forms there are several stages, several intermediate forms.
There are 6 types of neutrophils:
- myeloblasts;
- promyelocytes;
- myelocytes;
- metamyelocytes;
- band neutrophils;
- segmented neutrophils.
Most of all is in the blood of the latter. They are present in an amount of 40–75% of the total number of leukocytes. The number of rod-shaped neutrophils is significantly smaller, they can be 1–6%. The number of young cells does not reach 1%.
The need for additional examination
Despite their small numbers, basophils occupy an important place among leukocytes and are usually rapidly created by the body for a timely response to danger. By collapsing, these cells attract the attention of useful allies and at the same time create better conditions for blocking harmful agents, preventing them from capturing new areas. An increase in basophil levels may indicate a routine fight against an infection or parasite, as well as an allergy. In women, this indicator varies depending on the phase of the cycle and reflects the use of hormonal drugs.
Unfortunately, sometimes elevated basophil values indicate serious inflammation in autoimmune diseases, hemolytic anemia, liver damage, or insufficient synthesis of thyroid hormones. The most serious case is leukemia, in which blood cells are created in excessive numbers and the level of basophils can rise to 20%. Any serious disease cannot be diagnosed by one blood test result, or even by one type of test in principle. To avoid mistakes, the doctor is obliged to interview the patient and prescribe a whole range of suitable studies. Until these results are obtained, it is too early to draw conclusions.
Monocytes
Monocytes are cells of the immune system that are among the first to respond to the penetration of aggressors into the body. If the forces of local immunity could not contain the attack of bacteria, fungi or viruses, then it is monocytes that are the first to rush to protect health.
Monocytes are formed in the red bone marrow and released into the blood. There they begin to actively function, but this does not last long, only 2-3 days. Then, using their ability to move, they move beyond the vessels through special small pores between the cells and penetrate into the tissue. There, monocytes slightly change their structure and turn into macrophages - more effective phagocytes.