Rinsulin NPH, 100 IU/ml, suspension for subcutaneous administration, 3 ml, 5 pcs.
PC.
The dose of the drug is determined by the doctor individually in each specific case, based on the concentration of glucose in the blood. On average, the daily dose of the drug ranges from 0.5 to 1 IU/kg body weight (depending on the individual characteristics of the patient and blood glucose concentration).
IV administration of Rinsulin® NPH is contraindicated.
The temperature of the administered insulin should be at room temperature.
Usually the drug is injected subcutaneously into the thigh. Injections can also be made into the anterior abdominal wall, buttock, or into the shoulder area in the projection of the deltoid muscle. It is necessary to change injection sites within the anatomical region to prevent the development of lipodystrophies. When injecting insulin subcutaneously, care must be taken not to enter a blood vessel when injecting. After the injection, do not massage the injection site. Patients should be trained in the correct use of the insulin delivery device.
Before use, Rinsulin® NPH cartridges should be rolled between the palms in a horizontal position 10 times and shaken to resuspend the insulin until it takes the form of a homogeneous cloudy liquid or milk. Do not allow foam to appear, which may interfere with the correct dose.
Cartridges should be checked carefully. Do not use insulin if there are flakes in it after mixing, or if hard white particles have stuck to the bottom or walls of the cartridge, giving it a frozen appearance.
The design of the cartridges does not allow mixing their contents with other insulins directly in the cartridge itself. Cartridges are not intended to be refilled.
When using reusable pen cartridges, follow the manufacturer's instructions regarding inserting the cartridge into the pen and attaching the needle. The drug should be administered in accordance with the instructions of the syringe pen manufacturer.
After insertion, the needle must be unscrewed using the outer needle cap and disposed of immediately and safely. Removing the needle immediately after injection ensures sterility and prevents leakage, air entrapment, and possible needle clogging. Then you should put the cap on the pen.
When using multi-dose disposable syringe pens, it is necessary to mix the Rinsulin® NPH suspension in the syringe pen immediately before use. A properly mixed suspension should be uniformly white and cloudy.
Rinsulin® NPH in a pen cannot be used if it has been frozen.
When using prefilled multidose disposable pens for multiple injections, remove the pen from the refrigerator and allow the medication to reach room temperature before first use. You must follow the exact instructions for using the pen supplied with the drug.
Rinsulin® NPH pen and needle are for individual use only. The pen cartridge must not be refilled.
Needles should not be reused. To protect from light, the syringe pen should be closed with a cap. Do not store your used pen in the refrigerator. Store the drug in use at room temperature (15 to 25 °C) for no more than 28 days.
Using cartridges using reusable syringe pens
Cartridges with Rinsulin® NPH can be used with a reusable syringe pen:
— syringe pen Autopen Classic
3 ml 1 Unit (1–21 units) AN3810,
Autopen Classic
3 ml 2 Unit (2–42 units) AN3800) manufactured by Owen Mumford Ltd., UK;
— pen-injectors for administering insulin HumaPen® Ergo II and HumaPen® Luxury manufactured by Eli Lilly and Company, USA;
— syringe pen OptiPen® Pro 1 manufactured by Aventis Pharma Deutschland GmbH, Germany;
— BiomaticPen® syringe pen manufactured by Ipsomed AG, Switzerland.
It is necessary to follow the instructions for using syringe pens provided by the manufacturers.
Rinsulin® NPH
The drug is intended for subcutaneous administration.
The dose of the drug is determined by the doctor individually in each specific case based on the concentration of glucose in the blood.
Elderly patients using any insulin, including Rinsulin® NPH, are at increased risk of hypoglycemia due to the presence of comorbidities and simultaneous receipt of multiple medications. This may necessitate adjustment of the insulin dose.
Patients with renal or hepatic impairment are at increased risk of hypoglycemia and may require more frequent insulin dose adjustments and increased blood glucose monitoring.
Intravenous administration of Rinsulin® NPH is contraindicated.
The temperature of the administered insulin should be at room temperature.
Subcutaneous injections should be given in the upper arm, thigh, buttock or abdomen.
It is necessary to change injection sites within the anatomical region to prevent the development of lipodystrophies. When injecting insulin subcutaneously, care must be taken not to enter a blood vessel when injecting. After the injection, do not massage the injection site. Patients should be trained in the correct use of the insulin delivery device.
Before use, Rinsulin® NPH cartridges should be rolled between the palms in a horizontal position 10 times and shaken to resuspend the insulin until it takes the form of a homogeneous cloudy liquid or milk. Do not allow foam to appear, which may interfere with the correct dose.
Cartridges should be checked carefully. Do not use insulin if there are flakes in it after mixing, or if hard white particles have stuck to the bottom or walls of the cartridge, giving it a frozen appearance.
The design of the cartridges does not allow mixing their contents with other insulins directly in the cartridge itself. Cartridges are not intended to be refilled.
When using reusable pen cartridges, follow the manufacturer's instructions regarding inserting the cartridge into the pen and attaching the needle. The drug should be administered in accordance with the instructions of the syringe pen manufacturer.
After insertion, the needle must be unscrewed using the outer needle cap and disposed of immediately and safely. Removing the needle immediately after injection ensures sterility and prevents leakage, air entrapment, and possible needle clogging. Then you should put the cap on the pen.
4 When using multidose disposable syringe pens, it is necessary to mix the Rinsulin® NPH suspension in the syringe pen immediately before use. A properly mixed suspension should be uniformly white and cloudy. Rinsulin® NPH in a pen cannot be used if it has been frozen.
When using prefilled multidose disposable pens for multiple injections, remove the pen from the refrigerator and allow the medication to reach room temperature before first use. You must follow the exact instructions for using the pen supplied with the drug.
Rinsulin® NPH pen and needle are for individual use only. The pen cartridge must not be refilled.
Needles should not be reused.
To protect from light, the syringe pen should be covered with a cap.
Do not store your used pen in the refrigerator.
Rinsulin® NPH can be administered either alone or in combination with short-acting insulin (Rinsulin® R).
Store the drug in use at room temperature (15 to 25 °C) for no more than 28 days.
Using cartridges using reusable syringe pens
Cartridges with Rinsulin® NPH can be used with reusable syringe pens:
— Autopen Classic syringe pen (Autopen Classic 3 ml 1 Unit (1-21 units) AN3810, Autopen Classic 3 ml 2 Unit (2-42 units) AN3800) manufactured by Owen Mumford Ltd., UK;
— Pen-injectors for administering insulin HumaPen® Ergo II and HumaPen® Luxura and HumaPen® Savvio manufactured by Eli Lilly and Company, USA;
— Insulin syringe pen OptiPen Pro 1 manufactured by Aventis Pharma Deutschland GmbH, Germany;
— BiomaticPen® syringe pen manufactured by Ypsomed AG, Switzerland;
— Pen-injector for administering insulin individually RinsaPen® I produced by Ypsomed AG, Switzerland;
— Pen-injector for administering insulin individually RinsaPen® II produced by Ypsomed AG, Switzerland.
Carefully follow the instructions for using pens provided by their manufacturers.
INSTRUCTIONS FOR USING THE SYRINGE PEN
Rinastra®/Rinastra® II
(single multidose for multiple injections)
Ensuring asepsis during injection
Wash your hands with soap and water and select the injection site. Wipe the skin at the injection site with an alcohol wipe only after the insulin dose has been set in the syringe pen. Before injection, allow the alcohol to dry at the injection site.
Attention:
Before assembly, the syringe pen should be rolled between the palms in a horizontal position 10 times and shaken to resuspend the insulin until it takes the form of a homogeneous cloudy liquid or milk.
Assembly
A)
Hold the pen with one hand and remove the Cap by pulling it with the other hand.
Wipe the rubber membrane (Partition) with an alcohol wipe.
Note:
Using an alcohol wipe helps minimize the risk of infection.
B)
Select a needle from the set. Remove the protective sticker from the new Needle.
IN)
Using the External Attachment, install the needle directly onto the Cartridge Holder.
Tighten securely.
Attention:
Always use a new pen needle.
G)
Pull lightly to remove the Outer Attachment. Save the Outer Attachment for later removal of the used Needle.
Preparation
D)
Carefully remove the Inner Tip and discard. Hold the pen with the needle pointing up. Lightly tap the cartridge with your finger to help the air bubbles rise to the top. There may be some small bubbles left, but this is acceptable.
Note:
The needle becomes visible (exposed) as the Inner Tip is removed.
Checking the suitability of the syringe pen before injection
necessary to remove air from the needle.
Attention:
The suitability of the syringe pen must be checked before each injection.
E)
Scroll the Dosage Selector and set the dose to 2 units so that the number 2 coincides with the pointer in the Dosage window. A click will be heard as each unit is entered.
Note:
If the Dosage Selector has missed the required dose, simply turn it in the opposite direction to adjust the dose.
Attention:
Do not press the Start button while setting the dose.
AND)
While holding the Syringe Pen with the Needle pointing up, press the Start Button all the way. The dose selector will click when it reaches zero.
Check that a drop of liquid comes out of the needle. If this does not happen, repeat steps E and G, but no more than 6 times. If the drop still does not come out, remove the Needle (see step L) and repeat your steps starting from step B (selecting a new needle).
Attention:
To ensure that the dose is complete, you should always check that a drop of liquid comes out of the needle before each dose.
A slight “loss” of insulin is acceptable
Attention: Have you tested the pen with the dose set at 2 units to remove air from the needle? If not, go back to point "E".
Setting the dose
1)
Scroll the Dose Selector until the required dose aligns with the indicator in the Dosage Window.
For example, if you need a dose of 40 units, scroll the Dose Selector to 40 (as shown in the picture).
Attention:
You will not be able to select a dose greater than the number of units remaining in the cartridge. If the Dosage Selector does not scroll, this means that there is not enough medicine in the Syringe Pen. Throw away the Pen or administer the remaining dose units and use a new Pen to complete the required dose.
Dose administration
AND)
Make sure you have taken the required dose.
Clean the skin at the injection site with an alcohol wipe. Pinch the skin at the desired location and insert the needle under the skin in one continuous motion.
To avoid accidental needlestick injury:
- Pinch at least 2.5 cm of skin.
— DO NOT INSERT the needle at an angle towards the fingers.
TO)
Press the Start button until the value “0” coincides with the pointer in the Dosage window. Keep the button pressed and the pen at the injection site for 10 seconds after the stop click.
Attention:
Failure to follow these steps may result in the wrong dose being administered.
If you do not hold the pen at the injection site for the full 10 seconds, you may not receive the required dose of medication.
If insulin continues to leak from the needle after an injection, hold the needle in the skin longer for subsequent injections.
Disposal of a needle and storage of a syringe pen
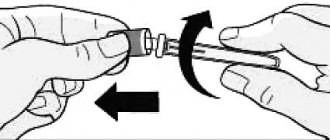
L)
Carefully push the Outer Attachment onto the needle until it stops. Unscrew the needle and discard it along with the Outer Attachment.
M)
Put on the Cap of the Syringe Pen and store the Syringe Pen until the next use.
Syringe pen care and disposal
— Keep the syringe pen away from direct sunlight.
— The syringe pen is intended for individual use and cannot be used by several people.
— Do not try to repair the Syringe pen yourself. Report the problem to the Complaints Authority specified in the Instructions for Medical Use.
An empty syringe pen should not be reused and must be destroyed.