Lantus SoloStar solution for subcutaneous injection 100 units/ml syringe pen 3 ml No. 5
Indications
- diabetes mellitus requiring treatment with insulin in adults, adolescents and children over 2 years of age.
pharmachologic effect
Insulin glargine is an analogue of human insulin, obtained by recombination of DNA from bacteria of the species Escherichia coli (strains K12) and is characterized by low solubility in a neutral medium.
In the composition of Lantus® SoloStar®, insulin glargine is completely soluble, which is ensured by the acidic reaction of the injection solution (pH 4). After injection into the subcutaneous fat, the acidic reaction of the solution is neutralized, which leads to the formation of microprecipitates, from which small amounts of insulin glargine are constantly released, providing a smooth (no peaks) profile of the concentration-time curve, as well as a prolonged effect of the drug.
Insulin glargine is metabolized to two active metabolites M1 and M2.
Connection with insulin receptors:
The kinetics of binding to specific insulin receptors of insulin glargine and its metabolites M1 and M2 are very close to those of human insulin, and therefore insulin glargine is capable of exerting biological effects similar to those of endogenous insulin.
The most important effect of insulin and its analogues, incl. and insulin glargine, is the regulation of glucose metabolism. Insulin and its analogues reduce blood glucose concentrations by stimulating glucose uptake into peripheral tissues (especially skeletal muscle and adipose tissue) and inhibiting glucose production in the liver. Insulin suppresses lipolysis in adipocytes and inhibits proteolysis, while simultaneously increasing protein synthesis.
The prolonged action of insulin glargine is directly related to the reduced rate of its absorption, which allows the drug to be used once a day. After subcutaneous administration, the onset of action occurs, on average, after 1 hour. The average duration of action is 24 hours, the maximum is 29 hours. The duration of action of insulin and its analogues, such as insulin glargine, can vary significantly from patient to patient or from patient to patient. and the same patient.
The effectiveness of the use of the drug Lantus® SoloStar® in children over 2 years of age with type 1 diabetes mellitus has been shown. In children in the age group 2-6 years, the incidence of hypoglycemia with clinical manifestations when using insulin glargine was numerically lower both during the day and at night compared with the use of isophane insulin (accordingly, an average of 25.5 episodes versus 33 episodes in one patient for one year).
During a five-year follow-up of patients with type 2 diabetes, there were no significant differences in the progression of diabetic retinopathy when treated with insulin glargine compared with isophane insulin.
Association with insulin-like growth factor 1 (IGF-1) receptors:
the affinity of insulin glargine for the IGF-1 receptor is approximately 5-8 times higher than that of human insulin (but approximately 70-80 times lower than that of IGF-1), while at the same time, compared to human insulin, the metabolites insulin glargine M1 and M2 have slightly less affinity for the IGF-1 receptor.
The total therapeutic concentration of insulin (insulin glargine and its metabolites) determined in patients with type 1 diabetes mellitus was markedly lower than the concentration required for half-maximal binding to IGF-1 receptors and subsequent activation of the mitogenic-proliferative pathway triggered through IGF-1 receptors. Physiological concentrations of endogenous IGF-1 can activate the mitogenic-proliferative pathway; however, therapeutic concentrations of insulin determined during insulin therapy, including treatment with Lantus® SoloStar®, are significantly lower than the pharmacological concentrations required to activate the mitogenic-proliferative pathway.
The ORIGIN (Outcome Reduction with Initial Glargine INtervention) study was an international, multicenter, randomized trial conducted in 12,537 patients at high risk of developing cardiovascular disease and with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or early stage diabetes mellitus type 2. Study participants were randomized (1:1) to receive insulin glargine (n=6264), titrated to achieve a fasting blood glucose (FBG) concentration of ≤5.3 mmol, or to receive standard care (n=6273). ).
The first endpoint of the study was the time to the first occurrence of cardiovascular death, the first occurrence of nonfatal myocardial infarction, or the first nonfatal stroke, and the second endpoint was the time to the first occurrence of any of the above complications or to a revascularization procedure (coronary, carotid, or peripheral arteries) , or before hospitalization for the development of heart failure.
Secondary endpoints were all-cause mortality and a composite measure of microvascular outcomes.
The ORIGIN study showed that treatment with insulin glargine, compared with standard hypoglycemic therapy, did not change the risk of cardiovascular events or cardiovascular mortality; There were no differences in any of the endpoints, all-cause mortality, or the composite microvascular outcome measure.
At baseline, the median HbA1c value was 6.4%. Median HbA1c values during treatment ranged from 5.9-6.4% in the insulin glargine group and 6.2-6.6% in the standard treatment group throughout the observation period.
In the group of patients receiving insulin glargine, the incidence of severe hypoglycemia was 1.05 episodes per 100 patient-years of therapy, and in the group of patients receiving standard hypoglycemic therapy it was 0.3 episodes per 100 patient-years of therapy. The incidence of non-severe hypoglycemia in the group of patients receiving insulin glargine was 7.71 episodes per 100 patient-years of therapy, and in the group of patients receiving standard hypoglycemic therapy - 2.44 episodes per 100 patient-years of therapy. In a 6-year study, 42% of patients in the insulin glargine group did not experience any hypoglycemia.
The median change in body weight from outcome at the last treatment visit was 2.2 kg higher in the insulin glargine group than in the standard care group.
Drug interactions
Oral hypoglycemic agents, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAO inhibitors, pentoxifylline, propoxyphene, salicylates and sulfonamide antimicrobial agents may enhance the hypoglycemic effect of insulin and increase the susceptibility to the development of hypoglycemia. These combinations may require dose adjustment of insulin glargine.
GCS, danazol, diazoxide, diuretics, glucagon, isoniazid, estrogens, gestagens (for example, in hormonal contraceptives), phenothiazine derivatives, somatotropin, sympathomimetics (for example, epinephrine (adrenaline), salbutamol, terbutaline), thyroid hormones, protease inhibitors, some antipsychotics (eg, olanzapine or clozapine) may reduce the hypoglycemic effects of insulin. These combinations may require dose adjustment of insulin glargine.
With simultaneous use of the drug Lantus® SoloStar® with beta-blockers, clonidine, lithium salts, ethanol, it is possible to either enhance or weaken the hypoglycemic effect of insulin.
Pentamidine, when combined with insulin, can cause hypoglycemia, which sometimes gives way to hyperglycemia.
When used simultaneously with drugs that have a sympatholytic effect, such as beta-blockers, clonidine, guanfacine and reserpine, there may be a decrease or absence of signs of adrenergic counterregulation (activation of the sympathetic nervous system) with the development of hypoglycemia.
Pharmaceutical interactions
When mixing Lantus® SoloStar® with other medicinal substances, incl. with other insulins, as well as dilution of the drug, the formation of a sediment or a change in the profile of the drug’s action over time is possible.
Dosage regimen
Lantus® SoloStar® should be administered subcutaneously 1 time/day at any time of the day, but every day at the same time.
In patients with type 2 diabetes mellitus, Lantus® SoloStar® can be used both as monotherapy and in combination with other hypoglycemic drugs.
Target values for blood glucose concentrations, as well as the dose and timing of administration or administration of hypoglycemic drugs should be determined and adjusted individually.
Dose adjustments may also be necessary, for example, if the patient changes in weight, lifestyle, changes in the timing of insulin dosing, or other conditions that may increase the susceptibility to developing hypo- or hyperglycemia. Any changes in insulin dosage should be made with caution and under medical supervision.
Lantus® SoloStar® is not the insulin of choice for the treatment of diabetic ketoacidosis. In this case, preference should be given to intravenous administration of short-acting insulin. In treatment regimens that include basal and prandial insulin injections, 40-60% of the daily insulin dose is usually administered as insulin glargine to meet basal insulin requirements.
In patients with type 2 diabetes mellitus taking oral hypoglycemic drugs, combination therapy begins with a dose of insulin glargine 10 units once a day and subsequently the treatment regimen is adjusted individually.
Monitoring blood glucose concentrations is recommended in all patients with diabetes.
Switching from treatment with other hypoglycemic drugs to Lantus® SoloStar®
When transferring a patient from a treatment regimen using intermediate-acting or long-acting insulin to a treatment regimen using Lantus® SoloStar®, it may be necessary to adjust the amount (doses) and time of administration of short-acting insulin or its analogue during the day or change the doses of oral hypoglycemic drugs .
In order to reduce the risk of developing hypoglycemia when transferring patients from a single daily administration of the drug Toujeo (insulin glargine 300 U/ml) to a single daily administration of the drug Lantus® SoloStar®, an initial dose of Lantus® SoloStar® is recommended, constituting 80% of the drug dose Toujeo, the use of which is being discontinued.
When transferring patients from a single daily administration of insulin-isophane to a single daily administration of the drug Lantus® SoloStar®, the initial doses of insulin usually do not change (i.e., the number of units of the drug Lantus® SoloStar® per day is used equal to the amount of IU insulin-isophane per day).
When transferring patients from twice daily administration of isophane insulin to a single administration of Lantus® SoloStar® before bedtime, in order to reduce the risk of hypoglycemia at night and early morning, the initial daily dose of insulin glargine is usually reduced by 20% (compared to daily dose of isophane insulin), and then it is adjusted depending on the patient's response.
When switching from human insulin to Lantus® SoloStar® and during the first weeks after it, careful metabolic monitoring (control of blood glucose concentrations) under medical supervision is recommended, with correction of the insulin dosage regimen if necessary. As with other human insulin analogues, this is especially true for patients who, due to the presence of antibodies to human insulin, require the use of high doses of human insulin. In such patients, when using insulin glargine, there may be a significant improvement in the response to insulin administration.
With improved metabolic control and the resulting increase in tissue sensitivity to insulin, it may be necessary to adjust the insulin dosage regimen.
Mixing and dilution
The drug Lantus® SoloStar® cannot be mixed with other insulins. Mixing may change the time/action ratio of Lantus® SoloStar® and may result in precipitation.
Special patient groups
Lantus® SoloStar® can be used in children over 2 years of age
.
Use in children under 2 years of age
has not been studied.
In elderly patients
with diabetes mellitus, it is recommended to use moderate initial doses, slowly increase them and use moderate maintenance doses. Elderly patients may have difficulty recognizing developing hypoglycemia.
Mode of application
The drug Lantus® SoloStar® is administered as a subcutaneous injection. The drug Lantus® SoloStar® is not intended for intravenous administration.
A long duration of action of insulin glargine is observed only when it is administered into the subcutaneous fat. IV administration of the usual subcutaneous dose may cause severe hypoglycemia.
Lantus® SoloStar® should be injected into the subcutaneous fat of the abdomen, shoulders or thighs. Injection sites should be alternated with each new injection within the recommended areas for subcutaneous administration of the drug.
As with other types of insulin, the extent of absorption, and therefore the onset and duration of action, may vary due to exercise and other changes in the patient's condition.
Lantus® SoloStar® is a clear solution, not a suspension. Therefore, resuspension before use is not required.
If the Lantus® SoloStar® syringe pen malfunctions, insulin glargine can be removed from the cartridge into a syringe (suitable for insulin 100 IU/ml) and the necessary injection can be made. In this case, the syringe should not contain residues of other medications.
Rules for use and handling of the SoloStar® pre-filled syringe pen
Before first use, the syringe pen must be kept at room temperature for 1-2 hours.
Before use, you should inspect the cartridge inside the syringe pen. It should only be used if the solution is clear, colorless, contains no visible solids, and has a consistency similar to water.
Empty SoloStar® syringe pens should not be reused and must be destroyed.
To prevent infection, the prefilled pen should only be used by one patient and not shared with another person.
Before using the SoloStar® syringe pen, you should carefully read the information on use.
Before each use, carefully attach a new needle to the pen and perform a safety test. Only needles compatible with SoloStar® should be used.
Special precautions must be taken to avoid needle-related accidents and the possibility of transmitting infection.
Under no circumstances should you use the SoloStar® syringe pen if it is damaged or if you are not sure that it will work properly.
You should always have a spare SoloStar® syringe pen available in case your existing SoloStar® syringe pen is lost or damaged.
If the SoloStar® syringe pen is stored in the refrigerator, it should be taken out 1-2 hours before the intended injection so that the solution reaches room temperature. Injecting chilled insulin is more painful.
The used SoloStar® syringe pen must be destroyed.
The SoloStar® syringe pen must be protected from dust and dirt. The outside of the SoloStar® syringe pen can be cleaned by wiping it with a damp cloth. Do not immerse, rinse or lubricate the SoloStar® syringe pen in liquid, as this can damage it.
The SoloStar® syringe pen accurately doses insulin and is safe to use. It also requires careful handling. Situations in which damage to the SoloStar® syringe pen may occur should be avoided. If you suspect damage to an existing SoloStar® syringe pen, you should use a new syringe pen.
Stage 1: Insulin control
It is necessary to check the label on the SoloStar® pen to ensure that it contains the appropriate insulin. For the drug Lantus®, the SoloStar® syringe pen is gray with a purple button for administering the injection. After removing the cap of the syringe pen, the appearance of the insulin contained in it is controlled: the insulin solution should be transparent, colorless, free of visible solid particles and resemble water in consistency.
Stage 2. Connecting the needle
It is necessary to use only needles that are compatible with the SoloStar® syringe pen. For each subsequent injection, always use a new sterile needle. After removing the cap, the needle must be carefully installed on the syringe pen.
Stage 3: Carrying out the safety test
Before each injection, a safety test must be performed to ensure that the pen and needle are working properly and that air bubbles have been removed.
Measure out a dose equal to 2 units.
The outer and inner needle caps must be removed.
With the syringe pen facing upward, gently tap the insulin cartridge with your finger so that all air bubbles are directed towards the needle.
Fully press the injection button.
If insulin appears at the tip of the needle, the pen and needle are working correctly.
If no insulin appears at the needle tip, then step 3 can be repeated until insulin appears at the needle tip.
Stage 4. Dose selection
The dose can be set to within 1 unit from the minimum dose (1 unit) to the maximum dose (80 units). If it is necessary to administer a dose greater than 80 units, 2 or more injections should be given.
The dosage window should show "0" after completion of the safety test. After this, the required dose can be set.
Stage 5. Dose administration
The patient should be informed about the injection technique by a healthcare professional.
The needle must be inserted under the skin.
The injection button must be pressed fully. It is held in this position for another 10 seconds until the needle is removed. This ensures that the selected dose of insulin is administered completely.
Stage 6: Removing and discarding the needle
In all cases, the needle should be removed and discarded after each injection. This prevents contamination and/or infection, air from entering the insulin container, and insulin leakage.
Special precautions must be taken when removing and discarding the needle. Recommended safety precautions for removing and disposing of needles (eg, one-hand capping technique) should be followed to reduce the risk of needle-related accidents and prevent infection.
After removing the needle, you should close the SoloStar® syringe pen with the cap.
Overdose
Symptoms:
An overdose of insulin can lead to severe and sometimes prolonged hypoglycemia, which threatens the patient's life.
Treatment:
episodes of moderate hypoglycemia are usually relieved by ingestion of rapidly digestible carbohydrates. It may be necessary to change the drug dosage regimen, diet, or physical activity. Episodes of more severe hypoglycemia, accompanied by coma, seizures or neurological disorders, require intramuscular or subcutaneous administration of glucagon, as well as intravenous administration of a concentrated dextrose solution. Long-term intake of carbohydrates and specialist supervision may be required, because recurrence of hypoglycemia is possible after visible clinical improvement.
Contraindications for use
- children under 2 years of age (lack of clinical data on use);
- hypersensitivity to insulin glargine or to any of the auxiliary components of the drug.
Carefully
the drug should be prescribed during pregnancy (the need for insulin may change during pregnancy and after childbirth).
Use in children
In children under 6 years of age, there is no clinical data on use.
Restrictions for children
Use with caution
Use in elderly patients
In elderly patients, progressive deterioration of renal function can lead to a persistent decrease in insulin requirements.
Restrictions for elderly patients
Use with caution
Use for liver dysfunction
In patients with severe liver failure, the need for insulin may be reduced due to a decrease in the ability to gluconeogenesis and a slowdown in the biotransformation of insulin.
Restrictions for liver dysfunction
Use with caution
Use during pregnancy and breastfeeding
Patients should inform their doctor about a current or planned pregnancy.
There are no randomized controlled clinical trials on the use of insulin glargine in pregnant women.
A large number of observations (more than 1000 pregnancy outcomes during retrospective and prospective observation) with post-marketing use of insulin glargine showed the absence of any specific effects on the course and outcome of pregnancy or on the condition of fetuses or the health of newborns.
In addition, a meta-analysis of eight observational clinical trials including women who used insulin glargine during pregnancy (n=331) was conducted to evaluate the safety of insulin glargine and isophane insulin in pregnant women with pre-existing or gestational diabetes mellitus. and insulin isophane (n=371). This meta-analysis found no significant differences in maternal or neonatal safety between insulin glargine and isophane insulin during pregnancy.
No direct or indirect evidence of the embryotoxic or fetotoxic effects of insulin glargine has been obtained from animal studies.
For patients with pre-existing or gestational diabetes mellitus, it is important to maintain adequate metabolic regulation throughout pregnancy to prevent adverse outcomes associated with hyperglycemia.
The drug Lantus® SoloStar® can be used during pregnancy according to clinical indications.
The need for insulin may decrease in the first trimester of pregnancy and, in general, increase in the second and third trimesters.
Immediately after birth, the need for insulin decreases rapidly (the risk of hypoglycemia increases). In these conditions, careful monitoring of blood glucose concentrations is essential.
Patients during breastfeeding may require adjustments to their insulin dosage regimen and diet.
Restrictions when breastfeeding
Use with caution
Restrictions during pregnancy
Use with caution
Use for renal impairment
In patients with impaired renal function, the need for insulin may decrease due to a weakening of its elimination processes. In elderly patients, progressive deterioration of renal function can lead to a persistent decrease in insulin requirements.
Restrictions for impaired renal function
Use with caution
Storage conditions
The drug should be stored out of the reach of children, protected from light at a temperature of 2° to 8°C; do not freeze; Shelf life: 3 years.
When storing Lantus® SoloStar® in the refrigerator, ensure that the containers do not come into direct contact with the freezer compartment or frozen packages.
Used SoloStar® disposable syringe pens should be stored in a place protected from light at a temperature not exceeding 30°C. The pre-filled SoloStar® syringe pen should not be refrigerated. Before first use, the Lantus® SoloStar® syringe pen must be kept at room temperature for 1-2 hours.
Terms of sale
The drug is available with a prescription.
special instructions
Lantus® SoloStar® is not the drug of choice for the treatment of diabetic ketoacidosis. In such cases, intravenous administration of short-acting insulin is recommended.
Due to limited experience with the use of Lantus® SoloStar®, it was not possible to evaluate its effectiveness and safety in the treatment of patients with impaired liver function or patients with moderate or severe renal failure.
In patients with impaired renal function, the need for insulin may decrease due to a weakening of its elimination processes. In elderly patients, progressive deterioration of renal function can lead to a persistent decrease in insulin requirements.
In patients with severe liver failure, the need for insulin may be reduced due to a decrease in the ability to gluconeogenesis and a slowdown in the biotransformation of insulin.
In case of ineffective control over the level of glucose in the blood, as well as in the presence of a tendency to the development of hypo- or hyperglycemia, before proceeding with the correction of the dosage regimen, you should check the accuracy of the prescribed treatment regimen, compliance with the instructions regarding the sites of drug administration and the correctness of the technique. subcutaneous injections, taking into account all factors that can affect the concentration of glucose in the blood.
Hypoglycemia
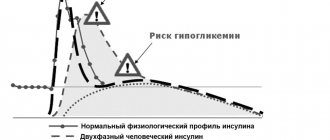
The time for the development of hypoglycemia depends on the action profile of the insulins used and may, therefore, change when changing the treatment regimen. Due to the increase in the time of entry into the body of long-acting insulin when using the drug Lantus® SoloStar®, one should expect a lower likelihood of developing nocturnal hypoglycemia, whereas in the early morning hours this likelihood is higher. If hypoglycemia occurs in patients receiving Lantus® SoloStar®, the possibility of slowing recovery from hypoglycemia due to the prolonged action of insulin glargine should be taken into account.
In patients in whom episodes of hypoglycemia may have particular clinical significance, incl. in patients with severe stenosis of the coronary arteries or cerebral vessels (risk of developing cardiac and cerebral complications of hypoglycemia), as well as patients with proliferative retinopathy, especially if they are not receiving photocoagulation treatment (risk of transient visual loss due to hypoglycemia), special precautions should be taken and close monitoring blood glucose level.
Patients should be warned about conditions in which the warning symptoms of hypoglycemia may decrease, become less severe, or be absent:
- with a noticeable improvement in the regulation of blood glucose concentrations;
- with the gradual development of hypoglycemia;
- in elderly patients;
- when transferring a patient from animal insulin to human insulin;
- with autonomic neuropathy;
- with long-term diabetes mellitus;
- if patients have mental disorders;
- concomitant treatment with other drugs.
Such situations may result in severe hypoglycemia (with possible loss of consciousness) before the patient is aware that he is developing hypoglycemia.
If normal or reduced levels of glycated hemoglobin are observed, the possibility of developing repeated unrecognized episodes of hypoglycemia (especially at night) must be considered.
Patients' compliance with the dosage regimen and diet, correct administration of insulin and knowledge of the warning signs of hypoglycemia help to significantly reduce the risk of developing hypoglycemia. Factors that increase the tendency to hypoglycemia, the presence of which require particularly careful monitoring and may require adjustment of the insulin dose:
- changing the site of insulin administration;
- increasing insulin sensitivity (for example, when eliminating stress factors);
- unusual, increased or prolonged physical activity;
- intercurrent diseases accompanied by vomiting, diarrhea;
- violation of diet and nutrition;
- missed meals;
- alcohol consumption;
- some uncompensated endocrine disorders (for example, hypothyroidism, insufficiency of the adenohypophysis or adrenal cortex);
- concomitant treatment with certain other drugs.
Intercurrent diseases
Intercurrent illnesses require more intensive control of blood glucose levels. In many cases, an analysis for the presence of ketone bodies in the urine is indicated, and adjustment of the insulin dosage regimen is also often required. The need for insulin often increases. People with type 1 diabetes should continue to regularly consume at least a small amount of carbohydrates, even when eating only small amounts or when unable to eat, or when vomiting. These patients should never stop taking insulin completely.
Side effect
The following undesirable reactions are presented by organ system (according to the MedDRA classification) in accordance with the following gradations of the frequency of their occurrence: very often (≥10%); often (≥1%; <10%); uncommon (≥0.1%; <1%); rarely (≥0.01%; <0.1%); very rare (<0.01%), frequency unknown (it is not possible to determine the frequency of adverse reactions from the available data).
From the side of metabolism:
very often - hypoglycemia. Hypoglycemia, the most common adverse reaction of insulin therapy, can occur when the dose of insulin is too high compared to the need.
Symptoms of hypoglycemia usually occur suddenly. However, often psychoneurological disorders associated with neuroglycopenia (feeling tired, unusual fatigue or weakness, decreased ability to concentrate, drowsiness, visual disturbances, headache, nausea, confusion or loss of consciousness, convulsive syndrome) are usually preceded by symptoms of adrenergic counterregulation (activation of sympathetic adrenal system in response to hypoglycemia): hunger, irritability, nervous agitation or tremor, anxiety, pale skin, “cold” sweat, tachycardia, palpitations (the faster hypoglycemia develops and the more severe it is, the more pronounced the symptoms of adrenergic counterregulation) .
Attacks of severe hypoglycemia, especially repeated ones, can lead to damage to the nervous system. Episodes of prolonged and severe hypoglycemia can threaten the lives of patients, because With increasing hypoglycemia, even death is possible.
From the immune system:
rarely - allergic reactions. Immediate allergic reactions to insulin are rare. Such reactions to insulin (including insulin glargine) or excipients may manifest as generalized skin reactions, angioedema, bronchospasm, decreased blood pressure or shock and may thus be life-threatening.
The use of insulin can cause the formation of antibodies to it. The formation of antibodies that cross-react with human insulin and insulin glargine occurs with equal frequency when using isophane insulin and insulin glargine. In rare cases, the presence of such antibodies to insulin may necessitate adjustment of the dosage regimen in order to eliminate the tendency to develop hypo- or hyperglycemia.
From the nervous system:
very rarely - dysgeusia.
From the side of the organ of vision:
rarely - visual impairment (significant changes in the regulation of glucose in the blood can cause temporary visual impairment due to changes in tissue turgor and the refractive index of the eye lens); retinopathy (long-term normalization of blood glucose reduces the risk of progression of diabetic retinopathy, however, insulin therapy, accompanied by sharp fluctuations in blood glucose concentrations, may be accompanied by a temporary worsening of diabetic retinopathy).
In patients with proliferative retinopathy, especially those not receiving photocoagulation treatment, episodes of severe hypoglycemia may lead to transient vision loss.
For the skin and subcutaneous tissues:
often - lipodystrophy (in 1-2% of patients). As with treatment with any other insulin preparations, lipodystrophy may develop at the injection site, which can slow down the local absorption of insulin. Uncommon: lipoatrophy. Constantly changing injection sites within areas of the body recommended for subcutaneous insulin administration may help reduce the severity of this reaction or prevent its development.
From the musculoskeletal system:
very rarely - myalgia.
General disorders and disorders at the injection site:
often - reactions at the injection site (3-4%) (redness, pain, itching, urticaria, swelling or inflammation). Most minor insulin injection site reactions usually resolve within a few days to a few weeks. Rarely - sodium retention, edema (especially if intensified insulin therapy leads to improvement of previously insufficient metabolic control).
Safety profile for patients under 18 years of age
, is generally similar to the safety profile for patients over 18 years of age. In patients under 18 years of age, reactions at the injection site and skin reactions (rash, urticaria) are relatively more common. There are no safety data in patients under 2 years of age.
Possible product names
- Lantus SoloStar solution s/c injected. 100 units/ml. syringe pen 3.0 ml No. 5
- LANTUS SOLOSTAR 100 UNITS/ML 3 ML No. 5 SYRINGE PEN
- LANTUS SOLOSTAR 100U/ML 3ML N5 SYRINGE PEN RR
- LANTUS SOLOSTAR RR D/PC.ENTERED. 100 UNITS/ML SPR/PEN 3 ML X5
- LANTUS SOLOSTAR SOLUTION FOR SC INTRODUCTION. 100 UNITS/ML SYRINGE PEN 3ML No. 5
- LANTUS SOLOSTAR 100 UNITS/ML SR D/SUBCUTANEOUS. ENTER 3ML SYRINGE PEN SOLOSTAR X5 (R)
- (Lantus SoloStar) Lantus SoloStar solution s/c injected. 100 units/ml. syringe pen 3.0 ml No. 5
Lantus® solostar® (Lantus® solostar®)
Lantus® SoloStar® should be administered subcutaneously 1 time/day at any time of the day, but every day at the same time.
In patients with type 2 diabetes mellitus, Lantus® SoloStar® can be used both as monotherapy and in combination with other hypoglycemic drugs. Target values for blood glucose concentrations, as well as the dose and timing of administration or administration of hypoglycemic drugs should be determined and adjusted individually.
Dose adjustments may also be necessary, for example, if the patient changes in weight, lifestyle, changes in the timing of insulin dosing, or other conditions that may increase the susceptibility to developing hypo- or hyperglycemia. Any changes in insulin dosage should be made with caution and under medical supervision.
Lantus® SoloStar® is not the insulin of choice for the treatment of diabetic ketoacidosis. In this case, preference should be given to intravenous administration of short-acting insulin. In treatment regimens that include basal and prandial insulin injections, 40-60% of the daily insulin dose is usually administered as insulin glargine to meet basal insulin requirements.
In patients with type 2 diabetes mellitus taking oral hypoglycemic drugs, combination therapy begins with a dose of insulin glargine 10 units once a day and subsequently the treatment regimen is adjusted individually.
Monitoring blood glucose concentrations is recommended in all patients with diabetes.
Switching from treatment with other hypoglycemic drugs to Lantus® SoloStar®
When transferring a patient from a treatment regimen using intermediate-acting or long-acting insulin to a treatment regimen using Lantus® SoloStar®, it may be necessary to adjust the amount (doses) and time of administration of short-acting insulin or its analogue during the day or change the doses of oral hypoglycemic drugs .
When transferring patients from a single daily administration of insulin-isophane to a single daily administration of the drug Lantus® SoloStar®, the initial doses of insulin usually do not change (that is, the number of units of the drug Lantus® SoloStar® per day is used equal to the number of IU insulin-isophane per day ).
When transferring patients from twice daily administration of isophane insulin to a single administration of Lantus® SoloStar® before bedtime in order to reduce the risk of hypoglycemia at night and early morning, the initial daily dose of insulin glargine is usually reduced by 20% (compared to the daily dose insulin isophane), and then it is adjusted depending on the patient's response.
Lantus® SoloStar® should not be mixed with other insulin preparations or diluted. It is necessary to ensure that the syringes do not contain residues of other medications. Mixing or diluting may change the time profile of insulin glargine.
When switching from human insulin to Lantus® SoloStar® and during the first weeks after it, careful metabolic monitoring (control of blood glucose concentrations) under medical supervision is recommended, with correction of the insulin dosage regimen if necessary. As with other human insulin analogues, this is especially true for patients who, due to the presence of antibodies to human insulin, require the use of high doses of human insulin. In such patients, when using insulin glargine, there may be a significant improvement in the response to insulin administration.
With improved metabolic control and the resulting increase in tissue sensitivity to insulin, it may be necessary to adjust the insulin dosage regimen.
Mixing and dilution
The drug Lantus® SoloStar® cannot be mixed with other insulins. Mixing may change the time/action ratio of Lantus® SoloStar® and may result in precipitation.
Special patient groups
Lantus® SoloStar® can be used in children over 2 years of age
.
Use in children under 2 years of age
has not been studied.
In elderly patients
with diabetes mellitus, it is recommended to use moderate initial doses, slowly increase them and use moderate maintenance doses.
Mode of application
The drug Lantus® SoloStar® is administered as a subcutaneous injection. The drug Lantus® SoloStar® is not intended for intravenous administration.
A long duration of action of insulin glargine is observed only when it is administered into the subcutaneous fat. IV administration of the usual subcutaneous dose may cause severe hypoglycemia. Lantus® SoloStar® should be injected into the subcutaneous fat of the abdomen, shoulders or thighs. Injection sites should be alternated with each new injection within the recommended areas for subcutaneous administration of the drug. As with other types of insulin, the extent of absorption, and therefore the onset and duration of action, may vary due to exercise and other changes in the patient's condition.
Lantus® SoloStar® is a clear solution, not a suspension. Therefore, resuspension before use is not required. If the Lantus® SoloStar® syringe pen malfunctions, insulin glargine can be removed from the cartridge into a syringe (suitable for insulin 100 IU/ml) and the necessary injection can be made.
Rules for use and handling of the SoloStar® pre-filled syringe pen
Before first use, the syringe pen must be kept at room temperature for 1-2 hours.
Before use, you should inspect the cartridge inside the syringe pen. It should only be used if the solution is clear, colorless, contains no visible solids, and has a consistency similar to water.
Empty SoloStar® syringe pens should not be reused and must be destroyed.
To prevent infection, the prefilled pen should only be used by one patient and not shared with another person.
Before using the SoloStar® syringe pen, you should carefully read the information on use.
Before each use, carefully attach a new needle to the pen and perform a safety test. Only needles compatible with SoloStar® should be used.
Special precautions must be taken to avoid needle-related accidents and the possibility of transmitting infection.
Under no circumstances should you use the SoloStar® syringe pen if it is damaged or if you are not sure that it will work properly.
You should always have a spare SoloStar® syringe pen available in case your existing SoloStar® syringe pen is lost or damaged.
If the SoloStar® syringe pen is stored in the refrigerator, it should be taken out 1-2 hours before the intended injection so that the solution reaches room temperature. Injecting chilled insulin is more painful. The used SoloStar® syringe pen must be destroyed.
The SoloStar® syringe pen must be protected from dust and dirt. The outside of the SoloStar® syringe pen can be cleaned by wiping it with a damp cloth. Do not immerse, rinse or lubricate the SoloStar® syringe pen in liquid, as this can damage it.
The SoloStar® syringe pen accurately doses insulin and is safe to use. It also requires careful handling. Situations in which damage to the SoloStar® syringe pen may occur should be avoided. If you suspect damage to an existing SoloStar® syringe pen, you should use a new syringe pen.
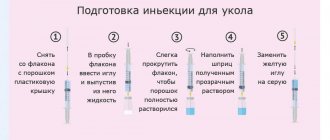
Stage 1: Insulin control
It is necessary to check the label on the SoloStar® pen to ensure that it contains the appropriate insulin. For Lantus, the SoloStar® syringe pen is gray with a purple button for injection. After removing the cap of the syringe pen, the appearance of the insulin contained in it is controlled: the insulin solution should be transparent, colorless, free of visible solid particles and resemble water in consistency.
Stage 2. Connecting the needle
It is necessary to use only needles that are compatible with the SoloStar® syringe pen. For each subsequent injection, always use a new sterile needle. After removing the cap, the needle must be carefully installed on the syringe pen.
Stage 3: Carrying out the safety test
Before each injection, a safety test must be performed to ensure that the pen and needle are working properly and that air bubbles have been removed.
Measure out a dose equal to 2 units.
The outer and inner needle caps must be removed.
With the syringe pen facing upward, gently tap the insulin cartridge with your finger so that all air bubbles are directed towards the needle.
Fully press the injection button.
If insulin appears at the tip of the needle, the pen and needle are working correctly.
If no insulin appears at the needle tip, then step 3 can be repeated until insulin appears at the needle tip.
Stage 4. Dose selection
The dose can be set to within 1 unit from the minimum dose (1 unit) to the maximum dose (80 units). If it is necessary to administer a dose greater than 80 units, 2 or more injections should be given.
The dosage window should show "0" after completion of the safety test. After this, the required dose can be set.
Stage 5. Dose administration
The patient should be informed about the injection technique by a healthcare professional.
The needle must be inserted under the skin.
The injection button must be pressed fully. It is held in this position for another 10 seconds until the needle is removed. This ensures that the selected dose of insulin is administered completely.
Stage 6: Removing and discarding the needle
In all cases, the needle should be removed and discarded after each injection. This prevents contamination and/or infection, air from entering the insulin container, and insulin leakage.
Special precautions must be taken when removing and discarding the needle. Follow recommended safety precautions for removing and disposing of needles (eg, one-handed capping technique) to reduce the risk of needle-related accidents and prevent infection.
After removing the needle, you should close the SoloStar® syringe pen with the cap.