Ignoring the symptoms, laziness and negligent attitude towards the treatment of type 2 diabetic disease (T2DM) have led to the fact that to the rather small number of patients with insulin-dependent type 1 diabetes (T1DM), every year a huge number of people with severe T2DM are added, who need to control their sugar levels. In the blood serum, injections of the insulin hormone are already required.
Moreover, diabetics with mild to moderate T2DM are recommended to take insulin injections during infectious diseases. Therefore, information about the drug Insulin Humalog will be useful to all diabetics, regardless of the type of diabetic disease.
The processing of glucose in the cells of the human body is impossible without the insulin hormone.
Domestic and foreign pharmacological companies produce a fairly wide range of different insulin preparations administered subcutaneously. They not only have different names and prices, but also different natures of origin, quality and duration of action. This article will talk about medications such as Insulin Humalog, Insulin Humalog Mix (50/50 and 75/25 options).
pharmachologic effect
Humalog® Mix 50 is a ready-made mixture consisting of a solution of insulin lispro 50% (a fast-acting analogue of human insulin) and a protamine suspension of insulin lispro 50% (an analogue of human insulin with an average duration of action).
The main effect of insulin lispro is the regulation of glucose metabolism.
In addition, it has anabolic and anti-catabolic effects on various tissues of the body. In muscle tissue, there is an increase in the content of glycogen, fatty acids, glycerol, increased protein synthesis and an increase in amino acid consumption, but at the same time there is a decrease in glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein catabolism and amino acid release.
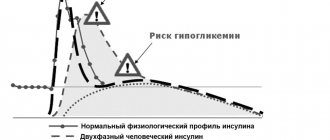
It has been shown that insulin lispro is equimolar to human insulin, but its effect is faster and lasts less. After subcutaneous injection of Humalog Mix 50, a rapid onset of action and an early onset of peak activity of insulin lispro are observed. The onset of action of the drug is approximately 15 minutes, which allows the drug to be administered immediately before meals (0-15 minutes before meals), compared to regular human insulin. After subcutaneous injection of Humalog Mix 50, a rapid onset of action and an early onset of peak activity of insulin lispro are observed. The action profile of insulin lispro protamine is similar to that of regular isophane insulin, with a duration of action of approximately 15 hours.
Humalog Mix 50 cartridges. with syringe pen 100 IU/ml 3 ml KwikPen No. 5
A country
France
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Insulin lispro biphasic
Compound
metacresol - 2.2 mg, liquid phenol - 1 mg, glycerol (glycerol) - 16 mg, protamine sulfate - 0.19 mg, sodium hydrogen phosphate heptahydrate - 3.78 mg, zinc oxide - qs for obtaining Zn2+ 30.5 mcg, water d/i - up to 1 ml ; hydrochloric acid solution 10% and/or sodium hydroxide solution 10% - qs to pH 7.0-7.8.
pharmachologic effect
Humalog® Mix 50 is a ready-made mixture consisting of a solution of insulin lispro 50% (a fast-acting analogue of human insulin) and a protamine suspension of insulin lispro 50% (an analogue of human insulin of medium duration). The main effect of insulin lispro is the regulation of glucose metabolism. In addition, it has anabolic and anti-catabolic effects on various tissues of the body. In muscle tissue, there is an increase in the content of glycogen, fatty acids, glycerol, increased protein synthesis and an increase in amino acid consumption, but at the same time there is a decrease in glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein catabolism and amino acid release. It has been shown that insulin lispro is equimolar to human insulin, but its effect comes faster and lasts less. After subcutaneous injection of Humalog Mix 50, a rapid onset of action and an early onset of peak activity of insulin lispro are observed. The onset of action of the drug is approximately 15 minutes, which allows the drug to be administered immediately before meals (0-15 minutes before meals), compared to regular human insulin. After subcutaneous injection of Humalog Mix 50, a rapid onset of action and an early onset of peak activity of insulin lispro are observed. The action profile of insulin lispro protamine is similar to that of regular isophane insulin, with a duration of action of approximately 15 hours.
Indications for use
- diabetes mellitus requiring insulin therapy.
Mode of application
Subcutaneously. The dose of Humalog® Mix 50 is determined by the doctor individually depending on the concentration of blood glucose. The insulin administration regimen is individual. The drug should be administered only subcutaneously. Intravenous administration of the drug Humalog® Mix 50 is unacceptable. The temperature of the administered drug should be at room temperature. Subcutaneous injections should be given in the upper arm, thigh, buttock or abdomen. Injection sites should be rotated so that the same site is used no more than approximately once a month. When administering Humalog Mix 50 subcutaneously, care should be taken to avoid the drug entering the lumen of blood vessels. After the injection, you should not massage the injection site. For recommendations on installing the cartridge into the device for administering Humalog Mix 50 and attaching a needle to it before administering the drug, read the manufacturer's instructions for the device for administering insulin. Strictly follow the instructions you have read. Preparation for administration Immediately before use, the cartridge with Humalog® Mix 50 should be rolled between your palms ten times and rocked, turning 180° also ten times to resuspend the insulin until it takes the form of a homogeneous cloudy liquid. Do not shake vigorously as this may cause foam, which may interfere with proper dosage delivery. To facilitate mixing, there is a small glass ball inside the cartridge. Do not use Humalog® Mix 50 if there are flakes in it after mixing. Dose administration1. Wash your hands.2. Select injection site.3. Prepare the skin at the injection site as directed by your physician.4. Remove the outer protective cap from the needle.5. Fix the skin by gathering it into a large fold.6. Insert the needle subcutaneously into the folded fold and perform the injection according to the instructions for using the syringe pen.7. Remove the needle and gently press the injection site with a cotton swab for a few seconds. Do not rub the injection site.8. Using the outer needle protective cap, unscrew the needle and discard it.9. Place the cap on the syringe pen. For the drug Humalog®Mix 50 in the KwikPen syringe pen. Before administering insulin, you should read the Guide for using the KwikPen syringe pen. Sufficient and well-controlled studies have not been conducted in pregnant women. Patients suffering from diabetes are advised to inform their doctor about an existing or planned pregnancy. During pregnancy, it is especially important to monitor the condition of patients receiving insulin therapy. Insulin requirements usually decrease during the 1st trimester and increase during the 2nd and 3rd trimesters. During and immediately after childbirth, the need for insulin may decrease sharply. In patients with diabetes mellitus during breastfeeding, adjustments in the dose of insulin, diet, or both may be required. Safety and effectiveness of Humalog® Mix 50 in patients under 18 years of age not studied.
Interaction
The hypoglycemic effect of Humalog® Mix 50 is reduced when co-administered with the following drugs: oral contraceptives, glucocorticosteroids, iodine-containing thyroid hormones, danazol, beta2-adrenergic agonists (for example, ritodrine, salbutamol, terbutaline), thiazide diuretics, chlorprothixene, diazoxide, isoniazid, nicotinic acid. acid, phenothiazine derivatives. The hypoglycemic effect of Humalog® Mix 50 is enhanced by: beta-blockers, ethanol and ethanol-containing drugs, anabolic steroids, fenfluramine, guanethidine, tetracyclines, oral hypoglycemic drugs, salicylates (for example, acetylsalicylic acid), sulfonamide antibiotics, some antidepressants (monoamine oxidase inhibitors), inhibitors ACE (captopril, enapril), octreotide, angiotensin II receptor antagonists. Beta-blockers, clonidine, reserpine can mask the manifestation of symptoms of hypoglycemia. The interaction of Humalog Mix 50 with other insulin preparations has not been studied.
Side effect
Hypoglycemia is the most common side effect that occurs with the administration of all insulin preparations, including Humalog® Mix 50. Severe hypoglycemia can lead to loss of consciousness and, in exceptional cases, death. Allergic reactions: patients may experience local allergic reactions in the form of redness , swelling or itching at the injection site. These minor reactions usually disappear within a few days or weeks. In some cases, these reactions may be caused by causes unrelated to insulin, such as skin irritation from the cleansing agent or improper injection. Systemic allergic reactions caused by insulin occur less frequently but are more severe. They can manifest as generalized itching, difficulty breathing, shortness of breath, decreased blood pressure, increased heart rate, and increased sweating. Severe cases of systemic allergic reactions can be life-threatening. In rare cases of severe allergy to Humalog Mix 50, immediate treatment is required. You may need to change insulin or carry out desensitization. With long-term use, lipodystrophy may develop at the injection site.
Contraindications
- hypoglycemia; - increased sensitivity to insulin or to one of the components of the drug. The safety and effectiveness of the drug Humalog® Mix 50 in patients under 18 years of age has not been studied.
Overdose
An overdose of insulin causes hypoglycemia, accompanied by the following symptoms: lethargy, increased sweating, tachycardia, pale skin, headache, trembling, vomiting, confusion. Under certain conditions, such as long-term illness or intensive control of diabetes, the warning signs of hypoglycemia may change. Mild hypoglycemia can usually be treated with oral glucose or sugar. Adjustments to your insulin dose, diet, or physical activity may be necessary. Correction of moderate hypoglycemia can be carried out using intramuscular or subcutaneous administration of glucagon, followed by oral carbohydrates. Severe conditions of hypoglycemia, accompanied by coma, convulsions or neurological disorders, are treated with intramuscular/subcutaneous administration of glucagon or intravenous administration of a concentrated solution of dextrose (glucose). After regaining consciousness, the patient must be given food rich in carbohydrates to avoid re-development of hypoglycemia. Continued carbohydrate intake and subsequent monitoring of the patient may be necessary as relapse of hypoglycemia may occur.
special instructions
Transferring a patient to another type or insulin preparation with a different trade name should occur under strict medical supervision. A change in insulin concentration, brand (manufacturer), type (Regular, NPH, etc.), species (animal, human, human insulin analog) and/or production method (DNA recombinant insulin or animal insulin) may require dose adjustment. For some patients, when switching from animal-derived insulin to human insulin, it may be necessary to adjust the dose. This may occur as early as the first injection of human insulin or gradually over several weeks or months after the transfer. Symptoms that are precursors of hypoglycemia during the administration of human insulin in some patients may be less pronounced or different from those observed during the administration of animal insulin. When blood glucose concentrations are normalized, for example, as a result of intensive insulin therapy, all or some of the symptoms that are precursors of hypoglycemia may disappear, of which patients should be informed. Precursor symptoms of hypoglycemia may change or be less severe with long-term diabetes mellitus, diabetic neuropathy, or treatment with drugs such as beta-blockers. The use of inappropriate doses or discontinuation of treatment, especially in patients with type 1 diabetes mellitus, can lead to hyperglycemia and diabetic ketoacidosis (conditions that are potentially life-threatening to the patient). The need for insulin may be reduced if there is insufficiency of the adrenal glands, pituitary gland or thyroid gland, or with renal or liver failure. With some illnesses or emotional stress, the need for insulin may increase. Adjustment of the insulin dose may also be required when increasing physical activity or changing the usual diet. Impact on the ability to drive vehicles and operate machinery During hypoglycemia, the patient's concentration and speed of psychomotor reactions may decrease. This may pose a hazard in situations where these abilities are particularly needed (for example, driving a vehicle or operating machinery). Patients should be advised to take precautions to avoid hypoglycemia while driving vehicles and operating machinery. This is especially important for patients with mild or absent warning signs of hypoglycemia or those with frequent hypoglycemia. In such cases, the doctor must evaluate the appropriateness of the patient driving a car and operating machinery.
Storage conditions
Cold +5
Dispensing conditions in pharmacies
On prescription
Directions for use and doses
The dose of Humalog® Mix 50 is determined by the doctor individually depending on the concentration of blood glucose. The insulin administration regimen is individual.
The drug should only be administered subcutaneously. Intravenous administration of Humalog® Mix 50 is unacceptable.
The temperature of the administered drug should be at room temperature. Subcutaneous injections should be given in the upper arm, thigh, buttock or abdomen. Injection sites should be rotated so that the same site is used no more than approximately once a month. When administering Humalog Mix 50 subcutaneously, care should be taken to avoid the drug entering the lumen of blood vessels. After the injection, do not massage the injection site.
At the beginning of treatment, the patient must be trained in injection techniques.
Side effects of the drug Humalog mix
Allergic reaction, injection site reaction, lipodystrophy, itching, rash, hypoglycemia. Hypoglycemia is the most common side effect of insulin therapy in patients with diabetes. Severe hypoglycemia can lead to loss of consciousness and, in some cases, death. Local allergies in patients may manifest themselves as redness, swelling or itching at the injection site. These minor reactions usually disappear after a few days or weeks. In some cases, these reactions may be due to factors other than insulin, such as detergents that irritate the skin or improper administration technique. Systemic allergy is very rare but is a potentially more serious side effect and is a generalized form of allergic reaction to insulin that can manifest as a rash over the entire body, shortness of breath, wheezing, decreased blood pressure, increased heart rate and sweating. Severe cases of generalized allergies are life-threatening.
special instructions
There are no sufficient and well-controlled studies in pregnant women. Patients suffering from diabetes are advised to inform their doctor about an existing or planned pregnancy. During pregnancy, it is especially important to monitor the condition of patients receiving insulin therapy. Insulin requirements usually decrease during the 1st trimester and increase during the 2nd and 3rd trimesters. During and immediately after childbirth, the need for insulin may decrease dramatically.
In patients with diabetes mellitus, adjustments in insulin dosage, diet, or both may be necessary during breastfeeding.
The safety and effectiveness of Humalog® Mix 50 in patients under 18 years of age have not been studied.
Transferring a patient to another type or insulin preparation with a different trade name should occur under strict medical supervision. A change in insulin concentration, brand (manufacturer), type (Regular, NPH, etc.), species (animal, human, human insulin analog) and/or production method (DNA recombinant insulin or animal insulin) may require dose adjustments.
The use of inadequate doses or discontinuation of treatment, especially in patients with type 1 diabetes, can lead to hyperglycemia and diabetic ketoacidosis (conditions that are potentially life-threatening for the patient). The need for insulin may be reduced if there is insufficiency of the adrenal glands, pituitary gland or thyroid gland, or with renal or liver failure. With some illnesses or emotional stress, the need for insulin may increase. Insulin dosage adjustments may also be necessary if you increase your physical activity or change your usual diet.
During hypoglycemia, the patient's concentration and speed of psychomotor reactions may decrease. This may pose a hazard in situations where these abilities are particularly needed (for example, driving a vehicle or operating machinery). Patients should be advised to take precautions to avoid hypoglycemia while driving vehicles and operating machinery. This is especially important for patients with mild or absent warning signs of hypoglycemia or those with frequent hypoglycemia. In such cases, the doctor must evaluate the appropriateness of the patient driving a car and operating machinery.
Indications, contraindications, side effects and other nuances
The instructions for the drug Insulin Humalog contain the following points:
- Indications: T1DM, T2DM, gestational diabetes, acute subcutaneous insulin resistance, uncorrectable postprandial hyperglycemia, accidentally acquired disease that complicates the course of diabetes, surgical operation for a diabetic patient.
- Contraindications: hyperglycemic conditions, increased individual sensitivity.
- Side effects - temporary insulin presbyopia of the lens, insulin swelling and typical hypoglycemic symptoms:
- headache;
- unnatural pallor of the skin;
- perspiration, increased profuse sweating;
- increased heart rate and heart rate;
- tremor of the limbs, muscle spasms, myoclonic twitching, paresthesia and various types of paresis;
- decrease in intellectual functions;
- sleep disorders;
- anxiety.
A glucagon injection must be included in a diabetic's personal first aid kit.
- Overdose – hypoglycemic precoma and coma. These conditions are treated with subcutaneous or intramuscular administration of glucagon. If there is no such drug or the desired effect is not obtained as a result of its use, an emergency injection of a ready-made glucose solution into a vein is performed.
- Cautions. In patients with problem kidneys and liver, during intense physical activity, in the absence of carbohydrates in food, as well as when treated with beta-blockers, sulfonamides or MAO inhibitors, when taking alcohol or alcohol-containing medications, the need for the drug may be underestimated. An increase in dose may be required during an infectious disease, with emotional distress, with a diet disorder, during therapy with thiazide diuretics, oral contraceptives, tricyclic antidepressants and glucocorticosteroids.
- Dosage. Lispro (Humalog) is injected under the skin, 4 to 6 times a day. The single dose, quantity and time of each injection are selected by the endocrinologist. A single injection with a dose higher than 40 units is allowed only in special cases. When switching to Lispro monotherapy from fast-acting porcine analogues, dosage adjustments may be required. During delivery and immediately after it, the dosage of the drug is recommended to be significantly reduced. A breastfeeding new mother with diabetes may also need dosage and/or diet adjustments.
- Features of storage and use. Insulin medications should be stored on the bottom shelf of the refrigerator. Before administration, the dose is “warmed up” by rolling it between the palms 10 to 20 times. You should also ensure that the injection does not enter a blood vessel.
Warning! When administering a cold drug, when alcohol gets under the skin, or simply due to its anabolic local effect, the formation of a cosmetic defect (lipohypertrophy) is possible, which reduces the absorption of the drug. Therefore, when making injections, you need to constantly change the location of the injections, and when punctures are in one area, for example, on the stomach, leave a distance of 1 cm between them.
Overdose of the drug Humalog mix, symptoms and treatment
Insulin overdose can cause hypoglycemia with associated symptoms, which may include apathy, confusion, palpitations, sweating, vomiting and headache. Hypoglycemia can develop due to excess insulin activity and a mismatch between food intake and energy expenditure, or both. Mild episodes of hypoglycemia can usually be treated with oral glucose. There may be a need for dose adjustment, changes in diet and physical activity. More severe episodes with coma, seizures, or neurological damage are treated with IM or SC administration of glucagon or concentrated IV glucose. Continued carbohydrate intake and evaluation may be necessary as hypoglycemia may recur after apparent clinical recovery.
Interactions of the drug Humalog mix
The need for insulin may increase when taking drugs with hyperglycemic activity, such as oral contraceptives, corticosteroids or thyroid hormone replacement therapy. Insulin requirements may be reduced by the use of oral hypoglycemic agents, salicylates, sulfonamides and some antidepressants (MAO inhibitors), ACE inhibitors and angiotensin II receptor blockers. Before using other medications in addition to Humalog Mix 25, you should consult your doctor.