Release form, composition and packaging
| Film-coated tablets | 1 tab. |
| zolpidem (as hemitartrate) | 10 mg |
15 pcs.
— cellular contour packages (1) — cardboard packs. 15 pcs. — contour cell packaging (2) — cardboard packs. pharmachologic effect
Zolpidem is an imidazopyridine hypnotic and is a selective agonist of the omega-1 benzodiazepine receptor subclass. It has a sedative effect, but when used in normal doses it does not have an anxiolytic, central muscle relaxant or anticonvulsant effect. Excites benzodiazepine receptors (omega) in the alpha subunit of GABA receptor complexes localized in the region of the IV plate of the sensory-motor zones of the cortex, reticular sections of the substantia nigra, visual hillocks of the ventral thalamic complex, pons, globus pallidus, etc. Interaction with omega receptors leads to to the opening of neuronal ionoform channels for chloride ions.
Shortens the time to fall asleep, reduces the number of night awakenings, increases the total duration of sleep and improves its quality. Extends stage II sleep and stages of deep sleep (III and IV). Does not cause drowsiness during the day.
Pharmacokinetics
Zolpidem is rapidly absorbed from the gastrointestinal tract. The time to reach Cmax in blood plasma after oral administration is 0.5-3 hours. Bioavailability is 70%, binding to plasma proteins is 92%. There is a linear relationship between the dose of the drug and its plasma concentrations. Metabolized in the liver to form three inactive metabolites, which are excreted by the kidneys (56%) and through the intestines (37%). The average T1/2 is 2.4 hours. Vd is 0.5±0.02 l/kg. Does not induce liver enzymes. In elderly people, plasma clearance may decrease without a significant increase in T1/2 (average 3 hours), while Cmax increases by 50%. In patients with severe renal impairment, clearance increases slightly. In patients with impaired liver function, bioavailability increases, T1/2 increases to 10 hours. It is excreted in small quantities in breast milk.
INSOMNIA
Insomnia is characterized not only by a lack of sleep, but also by an inability to get enough sleep, and may also include difficulty falling asleep, not getting enough sleep, or waking up early and then being unable to get back to sleep.
Pharmacological properties of the drug Zolpidem
Zolpidem has a hypnotic and sedative effect, belongs to the group of imidazopyridines, and is used for short-term relief of insomnia. Zolpidem is a potent, highly active ω-subtype agonist, also called the benzodiazepine receptor subtype 1 (BZ1) γ-aminobutyric acid type A (GABA A) chlorionophore-related receptor. It is believed that the ω1 subtype of the GABA A receptor is localized in the central nervous system mainly in the cerebellum, sensory-motor zone of the cerebral cortex, substantia nigra, superior colliculus, olfactory bulb, ventral part of the thalamic complex, pons and globus pallidus. The receptor complex is located on the membranes of neurons and functions by opening chloride channels, allowing chloride ions to pass through the membrane into the neuron. The result of this is hyperpolarization, which suppresses the neuron's firing. Unlike benzodiazepines, which non-selectively bind to GABA A receptors, ω1-, ω2- and ω3-subtype, zolpidem has pronounced selectivity for the ω1-subtype of GABA A receptors. Such selectivity is likely responsible for the relatively weak anticonvulsant, muscle relaxant and anxiolytic effects zolpidem when used in therapeutic doses and overall preservation of the structure of sleep phases. Absorbed quickly and completely, but bioavailability due to primary passage through the liver is 70%. The maximum concentration in blood plasma is achieved 30–120 minutes after oral administration. Concomitant use with food reduces the rate and extent of absorption. The volume of distribution in healthy volunteers after intravenous administration at a dose of 8 mg was 0.54 l/kg. It passes into breast milk in small quantities: 3 hours after oral administration at a dose of 20 mg, the amount of zolpidem in milk is 0.004–0.019% of the dose taken. 92% of zolpidem is bound to plasma proteins. Biotransformed in the liver with the formation of several inactive metabolites. The half-life is 2.6 hours (1.4–4.5 hours), may increase in the elderly and in patients with impaired liver or kidney function. 48–67% of zolpidem taken is excreted in urine, and 29–42% in feces. It is excreted in the form of metabolites; traces of unchanged zolpidem are detected in urine and feces. Safety and effectiveness in children have not been established.
Dosage
Orally (immediately before bedtime) in a single dose of 10 mg.
In elderly
or debilitated patients with impaired liver function
treatment begins with a dose of 5 mg.
If necessary (insufficient clinical effect) and the drug is well tolerated, the dose can be increased to 10 mg. The maximum daily dose is 10 mg. The course of treatment should not exceed 4 weeks. For transient insomnia,
the recommended course of treatment is 2-5 days, for
situational
insomnia - 2-3 weeks.
Very short periods of treatment do not require gradual withdrawal of the drug. In case of long-term use of the drug, to reduce the possibility of developing rebound insomnia, zolpidem should be discontinued gradually (first reducing the daily dose and then discontinuing the drug).
Use of the drug Zolpidem
In order to minimize the possibility of developing anterograde amnesia and other undesirable effects, zolpidem is taken only if it is possible to obtain 7-8 hours of sleep after its use. To promote sleep onset more quickly, zolpidem should be taken on an empty stomach. Usually prescribed at a dose of 10 mg orally at bedtime. The maximum daily dose for adults is up to 20 mg. Weakened patients, patients with impaired liver or kidney function, and elderly people are prescribed a reduced initial dose - 5 mg at bedtime, the dose is adjusted taking into account individual tolerance.
Overdose
Symptoms:
impaired consciousness (from confusion and lethargy to coma), ataxia, decreased blood pressure, respiratory depression.
Treatment:
induction of vomiting within 1 hour after an overdose, activated charcoal, if more than 1 hour has passed since the overdose (if conscious, by mouth, if unconscious, by tube), gastric lavage, symptomatic therapy. Flumazenil (a benzodiazepine receptor antagonist) is recommended as an antidote, but it should be remembered that antagonism with benzodiazepine receptors can lead to the development of seizures, especially in patients with epilepsy. Dialysis is ineffective.
Drug interactions
Not recommended combinations:
ethanol enhances the sedative effect of zolpidem.
Combinations that require caution when used:
- drugs that depress the central nervous system (neuroleptics, barbiturates, other hypnotics, anxiolytics/sedatives, antidepressants with sedative action, narcotic analgesics, antitussives of central action), antiepileptics, drugs for general anesthesia, antihistamines with sedative effect, hypotensive drugs of central action; baclofen; thalidomide; pizotifen - increased inhibitory effect on the central nervous system and risk of respiratory depression;
- buprenorphine - risk of respiratory depression;
- ketoconazole (a powerful inhibitor of CYP3A4) at a dose of 200 mg 2 times / day increases T1/2, AUC and reduces the clearance of zolpidem (possibly increasing the sedative effect of zolpidem);
- itraconazole (CYP3A4 inhibitor) - a slight, clinically insignificant change in the pharmacokinetics and pharmacodynamics of zolpidem.
Interactions to consider:
- rifampicin (CYP3A4 inducer) - accelerates metabolism, reduces the concentration and, as a consequence, the effectiveness of zolpidem.
Zolpidem drug overdose, symptoms and treatment
Manifested by severe ataxia, dysfunction of the cardiovascular system (bradycardia), visual impairment (diplopia), severe dizziness, drowsiness, nausea, respiratory failure, uncontrollable vomiting, inappropriate behavior, convulsions, loss of consciousness. Symptomatic and supportive treatment is carried out: monitoring of respiratory function and cardiovascular systems, neurological status, induction of vomiting or gastric lavage, administration of activated charcoal, refusal to use sedatives, even in a state of excitement. Flumazenil can be used to reverse the sedative effect of zolpidem and its depressant effect on breathing. Zolpidem is not eliminated by hemodialysis.
List of pharmacies where you can buy Zolpidem:
- Moscow
- Saint Petersburg
Side effects
Zolpidem is generally well tolerated.
Frequency of occurrence of adverse reactions: very often - more than 10%, often - more than 1% and less than 10%, infrequently - more than 0.1% and less than 1%), rarely - more than 0.01% and less than 0.1%, very rarely - less than 0.01% ( including isolated cases), the frequency is unknown (it is not possible to establish the frequency of occurrence based on the available data).
From the nervous system:
often - drowsiness, feeling of intoxication, headache, dizziness, increased insomnia, anterograde amnesia (the effects of amnesia may be associated with behavioral reactions), the risk of which increases in proportion to the dose, hallucinations, agitation, nightmares; infrequently - confusion, irritability; frequency unknown - disturbance of consciousness, dysphoria, aggressiveness, visual and auditory hallucinations, increased excitability, behavioral reactions, somnambulism, drug dependence (can develop even when using therapeutic doses), when discontinuing the drug - withdrawal syndrome or rebound insomnia, decreased libido, gait disturbance , ataxia, falls (mainly in elderly patients), addiction to the drug (decreased sedative and hypnotic effects when used for several weeks). Most mental side effects are paradoxical reactions.
From the digestive system:
often - diarrhea, nausea, vomiting, abdominal pain; frequency unknown - increased activity of liver enzymes.
From the musculoskeletal system:
frequency unknown - muscle weakness.
From the skin:
frequency unknown - rash, itching, urticaria, hyperhidrosis.
Allergic reactions:
frequency unknown - angioedema.
Other:
often - feeling tired; infrequently - diplopia.
results
- The average age of the patients was 83 years. 62% of patients were women. It was noted that among 3532 patients who started taking Z-drugs, 17% started taking them at a high dose.
- In patients receiving high-dose Z-drugs, compared with patients with sleep disorders who did not receive drugs, the risk of fractures increased by 1.67 times, femoral fracture by 1.96 times, falls by 1.33 times, and ischemic heart failure. stroke by 1.88 times.
- Similar results were observed when compared with non-sedative medications prescribed by primary care physicians.
- Minimal risks were observed when using doses ≤ 3.75 mg of zopiclone.
- There was no significant increase in risk with the use of Z-drugs compared with non-sedative drugs in terms of mortality, infections and venous thromboembolism.
- There were no significant differences in the incidence of adverse events between Z-drugs and benzodiazepines, excluding a 27% reduction in mortality for Z-drugs (hazard ratio, 0.73 [95% CI, 0.64 to 0.83]).
Contraindications
- acute and/or severe respiratory failure;
- severe acute or chronic liver failure;
— night apnea (including suspected);
- due to the presence of lactose in the composition: hereditary lactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome;
- pregnancy;
- lactation period;
- children's age (up to 18 years);
- hypersensitivity to zolpidem or other components of the drug.
Carefully :
myasthenia gravis, respiratory failure, mild to moderate liver failure, depression, alcoholism, drug addiction and other types of addiction.
special instructions
The persistence of insomnia for 7-14 days of treatment indicates the presence of primary mental disorders and/or disorders of the nervous system. Therefore, to identify these disorders, it is necessary to regularly reassess the patient's condition.
To reduce the risk of developing anterograde amnesia, patients should have conditions for uninterrupted 7-8 hours of sleep.
When using zolpidem, mental and behavioral (including paradoxical) reactions may occur (the risk of development is higher in elderly patients). If such reactions occur, zolpidem should be discontinued. After a course of taking it for several weeks, there may be some reduction in the sedative and hypnotic effects of zolpidem.
Use of zolpidem (especially long-term use) may lead to the formation of physical and/or mental dependence, the risk of which increases with increasing dose and duration of treatment, as well as in patients with a history of abuse of alcohol or other drugs and non-drug substances. Such patients should be closely monitored during treatment. However, dependence (extremely rarely) can also occur when using therapeutic doses and/or in patients without individual risk factors.
The combination of zolpidem with benzodiazepines increases the risk of developing drug dependence.
In elderly patients or with impaired liver function, a significant increase in T1/2 of zolpidem is possible, which can lead to accumulation of the drug when it is repeated. Based on the pharmacokinetics of zolpidem, drug accumulation is not expected in chronic renal failure.
When using zolpidem in elderly people, caution must be exercised due to the risk of developing pronounced sedative and/or muscle relaxant effects.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, you should refrain from engaging in potentially hazardous activities that require increased concentration (driving a car, working with machinery) and the speed of psychomotor reactions.
Zolpidem
In all cases, before using a sleeping pill, it is necessary, whenever possible, to establish the causes of sleep disturbances and carry out correction (including medication) of the underlying causes.
The persistence of insomnia for 7-14 days of treatment indicates the presence of primary mental disorders and/or disorders of the nervous system. Therefore, to identify these disorders, it is necessary to regularly assess the patient's condition.
Patients should always be warned about the recommended duration of treatment, which is determined by the type of insomnia (see section "Dosage and Administration").
Psychomotor disorders
The risk of developing psychomotor disorders, including impairment of the ability to drive vehicles, increases:
- when taking zolpidem less than 7-8 hours before performing activities that require concentration and rapid psychomotor reactions;
- when taking a dose exceeding the recommended dose;
- while taking zolpidem with other drugs that depress the central nervous system (CNS), ethanol, or drugs that increase the concentration of zolpidem in the blood;
Respiratory failure
Sleeping pills have the ability to depress the respiratory center; caution should be exercised when using the drug in patients with impaired respiratory function.
Liver failure
Zolpidem is contraindicated in patients with severe hepatic impairment, as it may contribute to the development of hepatic encephalopathy (see sections "Contraindications", "Dosage and Administration" and "Side Effects").
Use in elderly patients
When using zolpidem in elderly patients, caution must be exercised due to the risk of developing sedative and/or muscle relaxant effects, which can lead to falls with serious consequences for this group of patients (see section "Method of administration and dosage" subsection " Elderly patients").
Risk of accumulation
In elderly patients or patients with insufficient liver function, a significant increase in the half-life may occur (see section "Pharmacokinetics"), which may lead to accumulation of the drug upon repeated administration. Based on the pharmacokinetics of zolpidem, drug accumulation is not expected in patients with renal failure.
Psychotic illnesses
Hypnotics such as zolpidem are not recommended as primary treatment for psychotic illness.
Suicidal behavior and depression
Some epidemiological studies have shown an increased incidence of suicide and suicide attempts in patients with or without depression and who were taking benzodiazepines or other hypnotics, including zolpidem. A cause-and-effect relationship between the use of these drugs and the development of suicide and suicide attempts has not been established.
The use of zolpidem, like other sedative/hypnotic drugs, in patients with symptoms of depression requires extreme caution. These patients should be treated for depression but should not be given zolpidem alone. Manifestation of pre-existing latent depression is possible while taking zolpidem. Because these patients may be suicidal, they should be given the minimum amount of zolpidem required to avoid the possibility of the patient intentionally overdosing.
Because insomnia can be a symptom of depression, if insomnia persists, the patient should be re-evaluated to identify possible depression.
Decreased effectiveness (development of drug tolerance)
After a course of taking sedative/hypnotic drugs like zolpidem for several weeks, there may be some reduction in the hypnotic effect.
Formation of dependence on the drug
The use of sedative/hypnotic drugs like zolpidem can lead to physical and/or mental dependence. Dependence can also occur when using therapeutic doses and/or in patients without individual risk factors.
The risk of dependence increases with increasing dosage of the drug and duration of treatment, and is also higher in patients with a history of mental disorders, abuse of alcohol or other drugs and non-drug substances. Such patients should be especially closely monitored when taking sleeping pills.
If physical dependence on the drug develops, if the drug is abruptly stopped, the patient may develop “oricochete” syndrome, which will reduce his anxiety about the occurrence of such symptoms when stopping the drug. In the case of the use of short-acting sedative/hypnotics, symptoms of “paradoxical” reactions may occur
It is known that when using sedative/hypnotic drugs like zolpidem, other mental and “paradoxical” reactions may occur, such as anxiety, increased insomnia, agitation, irritability, aggressiveness, delirium, anger, nightmares, hallucinations, psychosis, behavioral abnormalities and other undesirable behavioral effects.
The appearance of these symptoms may be accompanied by the following behavioral disorders that are potentially dangerous for the patient or others:
- unusual behavior for the patient;
- self-injury or aggression towards others who are trying to prevent the patient from dangerous actions;
- automatic behavior with its subsequent amnesia.
If these effects occur, zolpidem should be discontinued. These effects are more likely to occur in older patients.
Somnambulism and associated challenging behavior
In some patients, benzodiazepines and related drugs can cause a syndrome of combined disorders of consciousness, behavior and memory of varying severity.
Patients who took zolpidem and were not fully awake experienced sleep walking and other associated complex behaviors: driving while half asleep, preparing and eating food, making phone calls, and engaging in sexual intercourse while not fully awake with amnesia for these activities.
Taking alcoholic beverages and other CNS depressants with zolpidem, as well as taking zolpidem in doses greater than the maximum recommended dose, appears to increase the risk of these behaviors. If the patient reports episode(s) of such behavior (for example, driving while drowsy), zolpidem should be discontinued due to the risk to both the patient and those around him (see Interactions with Other Drugs). means" subsection "With ethanol" and in the section "Side effects" subsection "Mental disorders").
Severe injuries
Due to its pharmacological properties, zolpidem can cause drowsiness and decreased level of consciousness, which can lead to patient falls and, as a result, serious injuries.
Patients with long QT syndrome
An in vitro study of the electrophysiological functions of cardiac muscle showed that, under experimental conditions, using very high concentrations and pluripotent stem cells, zolpidem can reduce the flow of potassium ions through hERG potassium channels. The possible consequences in patients with congenital long QT syndrome are unknown. As a precaution, the benefit/risk ratio should be carefully considered when treating patients with congenital long QT syndrome with zolpidem.
Use in children
The safety and effectiveness of zolpidem in children and adolescents under 18 years of age have not been established.
In an 8-week study in children and adolescents (ages 6–17 years) with insomnia associated with attention deficit hyperactivity disorder, the most common adverse reactions reported with zolpidem, compared with placebo, were psychiatric disorders and Nervous system disorders such as dizziness (23.5% vs 1.5%), headache (12.5% vs 9.2%) and hallucinations (7.4% vs 0%), see section " Method of administration and dosage."
For liver dysfunction
Contraindicated in acute or chronic liver failure.
With caution: mild to moderate liver failure.
In case of liver dysfunction
treatment begins with a dose of 5 mg.
If necessary (insufficient clinical effect) and the drug is well tolerated, the dose can be increased to 10 mg. The maximum daily dose is 10 mg. The course of treatment should not exceed 4 weeks. For transient insomnia,
the recommended course of treatment is 2-5 days, for
situational
insomnia - 2-3 weeks.
Use in old age
In elderly
patients,
treatment begins with a dose of 5 mg.
If necessary (insufficient clinical effect) and the drug is well tolerated, the dose can be increased to 10 mg. The maximum daily dose is 10 mg. The course of treatment should not exceed 4 weeks. For transient insomnia,
the recommended course of treatment is 2-5 days, for
situational
insomnia - 2-3 weeks.
Elderly patients may experience mental and behavioral (including paradoxical) reactions. If such reactions occur, zolpidem should be discontinued.
In elderly patients or with impaired liver function, a significant increase in T1/2 of zolpidem is possible, which can lead to accumulation of the drug when it is repeated.
When using zolpidem in elderly people, caution must be exercised due to the risk of developing pronounced sedative and/or muscle relaxant effects.
The main problem in the treatment of insomnia at present is not so much the insufficient effectiveness of the drugs used, but the insufficient safety of treatment with these drugs, especially for older people.
The main disadvantages of currently widely used benzodiazepine sleeping pills are: rapid loss of the hypnotic effect, the ability to impair memory, reduce psychomotor reactions, worsen well-being during the day, cause muscle weakness, and disrupt sleep structure. Due to some of these side effects, benzodiazepine hypnotics increase injury in older patients, and their long-term use is associated with a risk of dependence and withdrawal symptoms. This served as the basis for a search for hypnotics that do not belong to the group of benzodiazepines, which led to the creation and widespread introduction into practice of a new group of drugs that compare favorably with benzodiazepines in their greater safety.
The main difference between these new drugs is their ability to selectively interact with omega-1 benzodiazepine receptors, which ensures a limited range of unwanted side effects. One of these drugs is zolpidem, registered in Russia by the Sintelabo group under the trade name Ivadal.
Zolpidem is an imidazopyridine derivative with high affinity for the omega-1 subtype of the GABA-A receptor complex, which distinguishes it from benzodiazepine hypnotics, which stimulate all 3 omega receptor subtypes. Apparently, it is omega-1 receptors that mediate the hypnotic effect of all hypnotic drugs. It must be emphasized that the ability of zolpidem to bind only to omega-1 receptors is not absolute, but is considered as its distinctive pharmacological feature, which gives zolpidem certain advantages over other, less selective drugs. At the recommended therapeutic dose of 5-10 mg at night, zolpidem exhibits very weak anxiolytic, anticonvulsant and muscle relaxant activity. The virtual absence of a muscle relaxant effect is an important advantage of the drug, as it makes it possible to prescribe zolpidem to physically weakened patients.
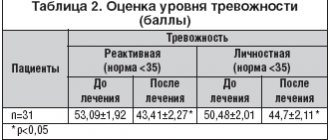
The hypnotic effect of zolpidem is manifested in facilitating sleep onset, reducing the frequency of night awakenings and prolonging sleep duration. Unlike benzodiazepine hypnotics, zolpidem does not disrupt sleep patterns. At the same time, it may prolong stages 3 and 4 of deep sleep into the early night hours with accompanying suppression of REM sleep. In the second half of the night, there is a tendency towards a compensatory increase in the rapid phase of sleep. These changes are most pronounced at the beginning of treatment and contribute to the restoration of normal relationships between the phases and stages of sleep (table).
Zolpidem is able to slow down the cyclic change of sleep phases (slow-rapid), which is associated with a feeling of subjective satisfaction with the quality of sleep. Although it is not yet known exactly how disruption of the sleep structure affects the state of health and mental processes, nevertheless, the ability of the drug to maintain it or bring it as close as possible to the physiological one should be considered as a fundamental property of an ideal sleeping pill.
Zolpidem has minimal effects on psychomotor performance and memory. The greatest changes in these parameters are observed only in the first few hours after taking the drug (which indicates the need to take the drug immediately before bedtime) and are not recorded after 6 or more hours. Studies have shown that in recommended doses (5-10 mg), zolpidem does not reduce memory, attention and other cognitive functions, does not cause daytime drowsiness, and does not impair psychomotor activity during the treatment period.
Zolpidem does not have a significant effect on respiratory function and does not worsen the condition of patients with chronic obstructive pulmonary diseases. However, there is evidence that the drug may increase apnea in patients prone to this condition. Therefore, zolpidem should not be used to treat insomnia caused by obstructive sleep apnea.
An important feature and advantage of zolpidem is the lack of development of addiction to the hypnotic effect of the drug for a long time: 3-6 months, and, according to some data, even for 1 year. There are isolated reports in the literature of the development of tolerance to zolpidem when taking the drug in high doses for a long time: from 2 months to several years. Patients gradually increased the dose of zolpidem to 70-400 mg per day. All of them, with the exception of one patient, had depressive disorders or personality disorders.
In clinical studies, discontinuation of long-term use of zolpidem was not accompanied by classic symptoms of withdrawal syndrome, although some deterioration in sleep was noted on the first (but not subsequent) night after discontinuation of therapy. Zolpidem is considered to have a low risk of dependence.
The pharmacokinetics of the drug is linear in the dose range from 5 to 20 mg. Zolpidem is rapidly absorbed when taken orally. Its bioavailability is approximately 70%. The drug does not accumulate in the body with repeated doses. Zolpidem actively binds to plasma proteins (92%). The volume of distribution is 0.54 l/kg. Biotransformation of the drug occurs in the liver under the action of enzymes of the cytochrome P450 system, mainly the CYP3A4 isoenzyme, with the formation of 3 inactive metabolites. Excretion of the drug in the form of metabolites is carried out by bile through the intestines and urine. Less than 1% of the total dose is excreted unchanged in the urine. In a very small amount (0.02% of the dose taken), zolpidem can be secreted into breast milk. The average half-life (T1/2) is 2.5 hours. In some categories of patients, certain changes in the pharmacokinetic parameters of zolpidem are observed. Thus, in patients over 70 years of age and even more so in patients suffering from cirrhosis of the liver, there is a decrease in the elimination of the drug and an increase in its concentration in the blood plasma. In this regard, it is recommended to start treatment of these groups of patients with a dose of 5 mg/day. In chronic renal failure, as a rule, there is no need for dose adjustment, despite the slight increase in T1/2 and volume of distribution of the drug noted in this condition.
Zolpidem is well tolerated by patients regardless of age. The incidence of side effects is 1-2%. The most common complaints are nausea, dizziness and drowsiness. Less commonly reported are headaches, nightmares and agitation. The use of zolpidem in elderly patients at a dose of 10 mg may lead to an increase in the frequency of these side effects, as well as the appearance of others, such as falls, confusion, and memory impairment.
There are reports in the literature of more than 300 cases of overdose of zolpidem taken in doses up to 1400 mg (average 190 mg). About half of the patients took zolpidem in combination with other, mainly psychotropic drugs or alcohol. An overdose of zolpidem is not associated with the development of any specific phenomena. The main symptom is drowsiness. First aid consists of gastric lavage or administration of activated carbon. If necessary, use an antagonist of benzodiazepine compounds - flumazenil (Anexat).
Some drugs taken together with zolpidem may enter into pharmacodynamic or pharmacokinetic interactions with it. Cimetidine, chlorpromazine and imipramine can increase the sedative effect of zolpidem, despite the lack of pharmacokinetic interactions with it. Rifampicin reduces the plasma concentration of zolpidem and thus reduces the pharmacodynamic effects of the drug. Ketoconazole increases the T1/2 half-life of zolpidem and enhances zolpidem-induced disturbances in psychomotor reaction. Fluconazole does not affect the pharmacokinetics of zolpidem. Studies in healthy subjects have not revealed a significant interaction between fluoxetine, sertraline and zolpidem, however, there are isolated clinical reports of hallucinations as a result of the possible interaction of zolpidem with sertraline, fluoxetine, desipramine, bupropion and venlafaxine. In some patients, an increase in prothrombin levels was noted with the combined use of zolpidem and warfarin. If it is necessary to combine zolpidem with drugs that have a depressant effect on the central nervous system, it is recommended to reduce its dose in order to prevent possible potentiating effects.
Based on pharmacodynamic capabilities and pharmacokinetic characteristics, zolpidem should be used immediately before bedtime at a dose of 10 mg, and when prescribed to elderly people or patients suffering from liver failure, at a dose of 5 mg. Each course of zolpidem (as well as other sleeping pills) should not exceed 4 weeks. The drug is contraindicated for the treatment of insomnia in patients with severe hepatic impairment, obstructive sleep apnea, acute respiratory failure or respiratory depression. Zolpidem should be administered with caution to patients suffering from depression. It is not recommended to use the drug during pregnancy and breastfeeding, since there is no complete information about the effects of zolpidem in these categories of patients. The drug is well tolerated, characterized by a relative absence of aftereffects, suppression of memory and other cognitive functions, as well as a low risk of developing dependence and withdrawal syndrome.
Thus, the introduction of zolpidem into clinical practice can be considered an important achievement that can significantly increase the safety of treatment of sleep disorders, including in elderly patients.