Manufacturer: Merck Sharp & Dohme Corp., USA
For the prevention of diseases caused by pneumococcal infection in children over 2 years of age and adults
Facts about pneumococcal disease
The review is based on a review of the Federal clinical guidelines from April 2015.
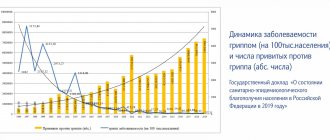
- According to WHO, pneumococcal infection is recognized as the most dangerous of all vaccine-preventable diseases.
- Pneumococci are bacteria that cause otitis media, bronchitis, pneumonia, meningitis, and sepsis.
- In Russia, out of 500 thousand cases of pneumonia per year, 76% of adults and up to 90% of children under 5 years of age have pneumococcal etiology!
- When examining children under 5 years of age hospitalized in Moscow hospitals for acute bacterial infections (sepsis, bacteremia, meningitis, pneumonia, acute otitis, sinusitis), culture of samples in 47% of cases revealed S. pneumoniae (“streptococcus pneumonia"), which confirms its leading role in the structure of infectious diseases.
- Carriage of streptococcus in the nasopharynx is asymptomatic in most cases, but in children of the first years of life it may be accompanied by a runny nose.
- Unfortunately, a large number of antibiotic-resistant forms of streptococcus are currently being formed.
- According to the WHO position, the only way to significantly influence the morbidity and mortality from pneumococcal infection is to reduce the level of antibiotic resistance.
- Currently in Russia, vaccination against pneumococcal infection is carried out with 3 drugs - Prevenar, Pneumovax 23, Synflorix. Your pediatrician will tell you in more detail which drug is most suitable for a particular case at your appointment.
- Currently, vaccination is carried out from 2 months of age until old age. In pediatrics, vaccination is most relevant for children who are frequently ill, weakened, or when planning hospitalizations (at least 2 weeks in advance); for children with concomitant pathologies: bronchial asthma, heart defects, etc.
In our clinic, we vaccinate children against pneumococcal infection with an individual approach to their condition, and, if necessary, prepare for vaccination.
Vaccine Pneumovax 23
Manufacturer Merck Sharp and Dome Corp., USA
Description of the drug
The Pneumovax 23 vaccine (polyvalent pneumococcal vaccine) includes the main types of pneumococci that cause diseases with severe clinical courses (otitis media, sepsis, meningitis, pneumonia). The Pneumovax 23 vaccine is a mixture of purified capsular polysaccharides of the 23 most common types of pneumococcus.
Vaccination against pneumococcal infection is aimed at preventing the disease, as well as reducing complications from pneumococcal infection and deaths.
Indications for use
Prevention of diseases caused by Streptococcus pneumoniae in children over 2 years of age and adults.
Directions for use and doses
The vaccine is administered subcutaneously or intramuscularly once, the vaccination dose is 0.5 ml for all ages.
Compatibility with other vaccines
The Pneumovax 23 vaccine can be administered simultaneously with all drugs from the national calendar of preventive vaccinations on the same day, in different parts of the body, with the exception of the BCG vaccine.
Administering several vaccines on the same day does not place an excessive burden on the immune system. All vaccines in the Russian national vaccination calendar are interchangeable.
Primary immunization is a single dose.
The combination of vaccination with Pneumovax 23 with vaccination against influenza is effective for the comprehensive prevention of acute respiratory infections and preparation for the autumn-winter season.
Side effects
The most common adverse reactions are soreness, swelling, induration and redness at the site where the vaccine was administered. It is also possible for the body temperature to rise to febrile levels. Side effects from the administration of the Pneumovax 23 vaccine are short-term in nature and do not require any specific therapy other than symptomatic treatment.
"Pneumovax 23" - vaccination against pneumococcal infection
Temporarily out of stock
Pneumococcal vaccine, polyvalent.
Manufacturer: Merck Sharp & Dohme Corp (USA)
Protects against diseases: a vaccine to prevent 23 types of pneumococcal infections.
Try on: children aged 2 years and older and adults over 50 years old with an increased risk of contracting pneumococcal infection.
Not included in the national vaccination calendar.
IMPORTANT: vaccinations with the Pneumovax 23 vaccine are not carried out in clinics
Advantages of the Pneumovax 23 vaccine:
- Vaccine against the 23 most common and invasive serotypes of Streptococcus pneumoniae.
- The vaccine is effective against pneumococcal serotypes that are the most common cause of antibiotic-resistant pneumococcal infections.
- A single vaccination is required.
- The introduction of the vaccine leads to the rapid appearance of specific antibodies, which after 3 weeks provide immunity to infection lasting 5-10 years.
- 57% protective effectiveness of vaccination against invasive infections caused by serotypes included in the vaccine in children over 6 years of age.
- Mild side effects.
pharmachologic effect
Vaccine for the prevention of diseases caused by Streptococcus pneumoniae.
Highly purified polyvalent vaccine. It is a purified polysaccharide of Streptococcus pneumoniae of 23 serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F , 23F, 33F. Causes the formation of immunity to the specified serotypes of Streptococcus pneumoniae. Immunity is acquired 10-15 days after a single vaccination and lasts for at least 5 years. After administration of this vaccine, seroconversion occurs in at least 90% of vaccinated individuals.
Indications for vaccination "Pneumovax 23"
Increased risk of developing pneumococcal disease, which is one of the leading causes of death worldwide and one of the leading causes of pneumonia, bacteremia, meningitis and otitis media in children over 2 years of age and in adults over 50 years of age.
special instructions
Despite the fact that there is no information about adverse effects on the fetus when using this vaccine during pregnancy, vaccination of pregnant women at risk is not recommended.
Vaccination using the Pneumovax® 23 vaccine will not protect against diseases caused by pneumococci of those capsular types that are not included in this vaccine.
If the Pneumovax® 23 vaccine is administered to persons receiving immunosuppressive therapy, the level of serum antibodies may be lower than expected and there may be an insufficient immune response to pneumococcal antigens (see subsection “Timing of vaccination”).
Intradermal administration may cause severe local adverse reactions.
As with any vaccine, vaccination with Pneumovax® 23 may not result in complete protection for all vaccinated individuals.
Vaccination with Pneumovax® 23 may not be effective in preventing infection resulting from a fracture of the base of the skull or leakage of cerebrospinal fluid into the external environment.
In patients whose condition requires penicillin (or other antibiotics) to prevent pneumococcal infection, such prophylaxis should not be stopped after vaccination with Pneumovax® 23.
Particular attention should be paid and appropriate precautions should be taken when administering Pneumovax® 23 to persons with severe forms of cardiovascular and/or pulmonary dysfunction.
Vaccination dates
For some diseases, the pneumococcal vaccine must be given at least two weeks before the planned splenectomy.
When planning cancer chemotherapy or other immunosuppressive therapy options (for example, in patients with Hodgkin's disease or those undergoing bone marrow or organ transplantation), the interval between vaccination and the start of immunosuppressive therapy should be at least two weeks. Vaccination during chemotherapy or radiation therapy should be avoided. The pneumococcal vaccine can be administered several months after completion of chemotherapy or radiation therapy for tumor diseases.
In Hodgkin's disease, after intensive chemotherapy (with or without radiation therapy), the immune response to vaccination may be reduced for two years or more.
Some patients experience significant improvement in their immune response within two years of completing chemotherapy or other immunosuppressive therapy (with or without radiation), especially as the interval between the end of treatment and pneumococcal vaccine administration increases.
Persons with asymptomatic or clinically evident HIV infection should be vaccinated as soon as possible after diagnosis.
The effect of the vaccine on the ability to drive and operate machines has not been studied.
Scheme and method of administration of the vaccine "Pneumovax 23"
In order to identify contraindications, the doctor conducts a survey and examination of the vaccinated person on the day of vaccination with mandatory thermometry.
Vaccinations are performed once. The vaccine is administered subcutaneously into the outer surface of the upper third of the shoulder. A single dose for all ages is 0.5 ml.
A single booster dose of Pneumovax 23 is recommended for persons 2 years of age and older who are at greatest risk of serious pneumococcal infections and those whose anti-pneumococcal antibody levels may decline rapidly, provided that at least five years have passed since The first dose of pneumococcal vaccine was administered. In children 10 years of age and younger who are considered to be at high risk for severe pneumococcal infections, revaccination with Pneumovax 23 may be considered three years after the previous dose of the vaccine.
Directions for use and doses
For intramuscular or subcutaneous administration! Do not administer intravenously or intradermally!
Before administration, the contents of the vial or syringe are checked for the presence of mechanical particles and color changes. The vaccine Pneumovax® 23 is a clear, colorless liquid. The Pneumovax® 23 vaccine is administered in a volume of 0.5 ml subcutaneously or intramuscularly (preferably into the deltoid muscle or the lateral surface of the mid-thigh), while taking the necessary precautions to avoid intravascular administration.
To prevent the transmission of infectious agents from one person to another, it is important to use a separate sterile syringe and needle for each individual patient.
No dilution or reconstitution of the drug is required.
The pre-filled syringe is for single use only. Inject the entire contents of the syringe.
Special patient groups
Children
The Pneumovax® 23 vaccine is not used in children under 2 years of age because children in this age group do not develop an effective immune response to the capsular antigens included in the polysaccharide vaccine.
Elderly patients
Clinical studies of the Pneumovax® 23 vaccine, which included persons aged 65 years and older, were conducted before and after the registration of this drug. In the largest of these studies, the safety of Pneumovax® 23 when administered to adults aged 65 years and older (n = 629) was compared with the safety of Pneumovax® 23 when administered to adults aged 50 to 64 years (n = 379). . Participants in this study were outpatients, and the prevalence of age-related chronic diseases was as expected. Clinical data did not allow us to identify an increased frequency and severity of adverse reactions in people over the age of 65 years compared to those in patients from the group of 50-64 years. However, since the tolerance of older people to medical interventions may not be the same as that of younger patients, a higher frequency and/or greater severity of reactions in some older people cannot be ruled out.
Post-marketing reports have been received noting that in some frail elderly people with multiple comorbidities, severe adverse events and worsening of the clinical course of existing diseases occurred after vaccination.
Compatibility with other vaccines
The pneumococcal vaccine Pneumovax 23 can be given at the same time as the flu vaccine (which is given in the other arm). Such administration does not lead to an increase in the frequency of side effects or a decrease in the intensity of the immune response to the administration of each of the vaccines.
The pneumococcal vaccine can be administered simultaneously (on the same day) with other vaccines (except for vaccines to prevent tuberculosis) in different parts of the body using different syringes.
The Pneumovax 23 vaccine can be administered no earlier than 4 weeks after Zostavax vaccination.
Preparing a child for vaccination
For successful immunization and minimizing side effects, the baby must be prepared in advance and follow some rules before and after vaccination:
- It is necessary to undergo an examination by a pediatrician and neurologist in advance. Possible reasons for delaying vaccination include: acute viral and bacterial diseases, severe anemia, high fever, etc. After eliminating the acute form of the disease, immunization is carried out according to the course;
- Avoid hypothermia and overheating;
- Prepare in advance for a possible increase in temperature (have paracetamol or ibuprofen at home). Fever is a predicted response to vaccination;
- After the injection, remain under medical supervision for at least 30 minutes.
All the vaccines we use comply with WHO standards, which guarantees their effectiveness, safety and reduced risk of side effects.
Contraindications
Only a doctor can decide whether PNEUMOVAX 23 is suitable for vaccination
Pneumovax 23 is contraindicated if there is a history of an allergic reaction to any component of the vaccine.
Vaccination with Pneumovax 23 is contraindicated in the following cases:
- Hypersensitivity to any component of the vaccine, including neomycin; symptoms of hypersensitivity to previous vaccine administration.
- Acute infectious and non-infectious diseases, exacerbation of chronic diseases are temporary contraindications for vaccinations, except in cases where, in the opinion of a doctor, delaying vaccination entails an even greater risk.
- Vaccination during chemotherapy or radiation therapy should be avoided.
Pneumococcal vaccine, polyvalent
Manufacturer: Merck Sharp & Dohme Corp, USA.
Protects against diseases: a vaccine to prevent 23 types of pneumococcal infections.
Try on: children aged 2 years and older and adults over 50 years old with an increased risk of contracting pneumococcal infection.
Not included in the national vaccination calendar.
IMPORTANT: vaccinations with the Pneumovax 23 vaccine are not carried out in clinics
How to help your young child feel comfortable during vaccinations
Vaccination works better when the baby is calm and not afraid. To avoid scaring your child, try the following:
- Distract and calm your baby, hug him, talk softly to him
- Be calm, confident, smile.
- Maintain eye contact with your child.
- Communicate with your child, show that you are nearby and everything is fine.
- Let your child hold a favorite toy or blanket.
- Ask your doctor if you can hold your baby on your lap and gently rub his back during vaccination.
- Be sure to praise your child after vaccination, tell him how great he is and how proud you are of him. Support your baby, even if he couldn't help but cry.
Reviews
Pneumovax 23 is widely used, so you can easily find many reviews from people who have used it. Most reviews are positive, and patients claim that they tolerated this vaccination well and then did not get sick with pneumococcal infection.
But there are also negative comments that there were complications with the drug. You should not rely only on the opinions of other people, because various side effects are associated only with individual intolerance to the vaccine; in order to make a final decision about whether you need this vaccination or not, consult your doctor.