Pharmacological properties
Pharmacodynamics.
Lornoxicam, an NSAID with analgesic and anti-inflammatory properties, belongs to the oxicam class. The mechanism of action of lornoxicam is mainly associated with inhibition of prostaglandin synthesis (inhibition of the COX enzyme), which leads to desensitization of peripheral nociceptors and inhibition of inflammation. a central effect on nociceptors is also suggested, which is not associated with anti-inflammatory effects. Lornoxicam does not affect vital signs (for example, body temperature, respiratory rate, heart rate, blood pressure, ECG, spirometry). The analgesic properties of lornoxicam were successfully demonstrated in several clinical studies during the development of the drug. Due to local gastrointestinal irritation and systemic ulcerogenic effects associated with inhibition of prostaglandin (PG) synthesis, the use of lornoxicam and other NSAIDs often leads to the development of gastrointestinal complications.
Pharmacokinetics. Absorption. Lornoxicam is quickly and almost completely absorbed from the gastrointestinal tract. Cmax is achieved 1–2 hours after taking the drug. The bioavailability of lornoxicam is 90–100%. No first pass effect was noted. The average half-life is 3–4 hours.
When co-administering lornoxicam with food, Cmax is reduced by approximately 30% and Tmax is increased from 1.5 to 2.3 hours. Absorption of lornoxicam (calculated according to the area under the concentration/time pharmacokinetic curve (AUC)) may be reduced by up to 20%.
Lornoxicam 8 mg powder for injection is intended for intravenous and intramuscular administration. Cmax in blood plasma after intramuscular administration of the drug is achieved after 0.4 hours. Bioavailability (calculated by AUC) after intramuscular administration of the drug is 97%.
Distribution. In blood plasma, lornoxicam is unchanged and in the inactive form of its hydroxylated metabolite. The binding of lornoxicam to plasma proteins is 99% and does not depend on its concentration.
Biotransformation. Lornoxicam is extensively metabolized in the liver by hydroxylation, initially to the inactive 5-hydroxylornoxicam. Lornoxicam undergoes biotransformation with the participation of cytochrome CYP 2C9. The metabolism of this enzyme due to genetic polymorphism may be slow or intense in different individuals, which may lead to a marked increase in plasma levels of lornoxicam in individuals with poor metabolism. The hydroxylated metabolite has no pharmacological activity. Lornoxicam is completely metabolized. Approximately ⅔ is excreted through the liver and 1/3 through the kidneys as an inactive compound.
In animal model studies, lornoxicam did not induce liver enzymes. Based on the results of clinical studies, there is no evidence of accumulation of lornoxicam after repeated administration in recommended doses. These results were confirmed by data from monitoring the safety and effectiveness of drugs for 1 year of the study.
Excretion. Half-life of the original substance is 3–4 hours. After oral administration, about 50% is excreted in the feces and 42% through the kidneys, mainly in the form of 5-hydroxylornoxicam. T½ of 5-hydroxylornoxicam is about 9 hours after parenteral administration of the drug 1 or 2 times a day.
In elderly patients (over 65 years of age), clearance is reduced by 30–40%. Apart from decreased clearance, there are no significant changes in the kinetic profile of lornoxicam in the elderly.
There is no significant change in the kinetic profile of lornoxicam in patients with renal or hepatic impairment, with the exception of accumulation in persons with chronic liver disease, after 7 days of therapy using daily doses of 12 and 16 mg.
Buy Xefocam film-coated tablets 8 mg No. 10 in pharmacies
INSTRUCTIONS for medical use of the drug XEFOCAM
Registration number
Trade name of the drug Xefocam
International nonproprietary name Lornoxicam
Dosage form Film-coated tablets
Composition per 1 tablet for a dosage of 4 mg. Active substance: lornoxicam - 4 mg. Excipients: magnesium stearate 2.0 mg, povidone (K25) 5.0 mg, croscarmellose sodium 10.0 mg, cellulose 85.0 mg, lactose monohydrate 94.0 mg. Shell: macrogol (6000) 1.2 mg, titanium dioxide E-171 2.4 mg, talc 4.8 mg, hypromellose 8.4 mg.
Composition per 1 tablet for a dosage of 8 mg. Active substance: lornoxicam - 8 mg. Excipients: magnesium stearate 2.0 mg, povidone (K25) 5.0 mg, croscarmellose sodium 10.0 mg, cellulose 85.0 mg, lactose monohydrate 90.0 mg. Shell: macrogol (6000) 0.8 mg, titanium dioxide E-171 1.6 mg, talc 3.2 mg, hypromellose 5.6 mg.
Description White to yellowish oblong film-coated tablets, inscribed with “LO4” (4 mg dosage) and “LO8” (8 mg dosage) indented.
Pharmacotherapeutic group Non-steroidal anti-inflammatory drug (NSAID).
ATX code: M01AC05
Pharmacological action Has a pronounced analgesic and anti-inflammatory effect. Lornoxicam has a complex mechanism of action, which is based on the suppression of prostaglandin synthesis due to inhibition of the activity of cyclooxygenase isoenzymes. In addition, lornoxicam inhibits the release of oxygen free radicals from activated leukocytes. The analgesic effect of lornoxicam is not associated with narcotic effects. The drug XEFOCAM does not have an opiate-like effect on the central nervous system and, unlike narcotic analgesics, does not depress respiration and does not cause drug dependence. Pharmacokinetics Lornoxicam is rapidly and almost completely absorbed from the gastrointestinal tract after oral administration. In this case, maximum plasma concentrations are reached after approximately 1-2 hours. Eating reduces the maximum concentration (Cmax) by 30% and increases the time to reach the maximum concentration (Tmax) to 2.3 hours. The absolute bioavailability of lornoxicam is 90-100%. Lornoxicam is present in plasma mainly unchanged and, to a lesser extent, in the form of a hydroxylated metabolite, which has no pharmacological activity. The binding of lornoxicam to plasma proteins, predominantly the albumin fraction, is 99% and does not depend on its concentration. The half-life averages 4 hours and does not depend on the concentration of the drug. Lornoxicam is completely metabolized in the liver. CYP2C9 is involved in metabolism. Approximately 1/3 of the metabolites are excreted from the body by the kidneys and 2/3 by the bile. In elderly people, as well as in patients with renal or hepatic insufficiency, no significant changes in the pharmacokinetics of lornoxicam were found.
Indications for use : Short-term treatment of pain of various origins. Symptomatic treatment of rheumatic diseases (rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, articular syndrome with exacerbation of gout, rheumatic soft tissue damage).
Contraindications - Known hypersensitivity/allergy to lornoxicam or to one of the components of the drug; - complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history); - hemorrhagic diathesis or bleeding disorders, as well as those who have undergone operations associated with the risk of bleeding or incomplete hemostasis; — the period after coronary artery bypass grafting; - erosive and ulcerative changes in the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding; cerebrovascular or other bleeding; - recurrent gastric ulcer or repeated gastrointestinal bleeding; - Gastrointestinal bleeding associated with a history of taking NSAIDs; - inflammatory bowel diseases (Crohn's disease, ulcerative colitis) in the acute phase; - decompensated heart failure; - severe liver failure or active liver disease; - severe renal failure (serum creatinine level more than 300 µmol/l), progressive kidney disease, confirmed hyperkalemia, hypovolemia or dehydration; — pregnancy, breastfeeding period; - childhood .
With caution Erosive and ulcerative lesions and bleeding from the gastrointestinal tract (history), moderate renal failure, conditions after surgical interventions, age over 65 years, body weight less than 50 kg, coronary heart disease, heart failure, cerebrovascular diseases, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial diseases, smoking, creatinine clearance less than 60 ml/min, a history of ulcerative lesions of the gastrointestinal tract, the presence of H. pylori infection, long-term use of NSAIDs, alcoholism, severe somatic diseases, simultaneous use of oral corticosteroids (including prednisolone), anticoagulants ( including warfarin), antiplatelet agents (including clopidogrel), selective serotonin reuptake inhibitors (including citalopram, fluoxetine, paroxetine, sertraline).
Method of administration and dosage For oral administration: for severe pain, the recommended dose is 8-16 mg/day, divided into 2-3 doses. The maximum daily dose is 16 mg. For inflammatory and degenerative rheumatic diseases, the recommended starting dose is 12 mg. The standard dose is 8-16 mg per day, depending on the patient's condition. The duration of therapy depends on the nature and course of the disease. XEFOCAM tablets are taken orally before meals with a glass of water. For diseases of the gastrointestinal tract, patients with impaired renal or liver function, and elderly people (over 65 years of age), it is recommended to use the minimum effective dose of the drug in the shortest possible short course. The maximum daily dose is 12 mg, divided during the day into 3 doses of 4 mg.
Side effects From the gastrointestinal tract and liver: dyspepsia, abdominal pain, dry mouth, stomatitis, nausea, vomiting, heartburn, diarrhea; esophagitis, gastritis, erosive and ulcerative lesions of the mucous membrane of the stomach and intestines, incl. with perforation and bleeding, constipation, flatulence, melena, impaired liver function, increased levels of liver transaminases; From the nervous system: headache, dizziness, drowsiness, sleep disturbances, depression, agitation, tremor, aseptic meningitis, paresthesia; On the part of the skin and subcutaneous fat: edematous syndrome, ecchymosis, skin rash, itching, urticaria, alopecia, Stevens-Johnson syndrome, Lyell's syndrome; From the urinary system: dysuria, decreased glomerular filtration, interstitial nephritis, glomerulonephritis, papillary necrosis, nephrotic syndrome, peripheral edema, acute renal failure; From the senses: tinnitus, blurred vision; From the cardiovascular system: development or worsening of heart failure, tachycardia, increased blood pressure; From the hematopoietic organs and hemostasis system: agranulocytosis, leukopenia, anemia, thrombocytopenia, increased bleeding time; From the respiratory system: pharyngitis, rhinitis, dyspnea, cough, bronchospasm; Other: anorexia, increased sweating, changes in body weight, arthralgia, myalgia, angioedema.
Overdose Symptoms: the side effects of XEFOCAM described above may increase. Treatment: symptomatic. Taking activated carbon immediately after taking XEFOCAM may help reduce the absorption of this drug. Antiulcer drugs can be used to prevent damage to the mucous membrane.
Interaction with other drugs The simultaneous use of XEFOCAM and cimetidine increases the concentration of lornoxicam in plasma. No interactions with ranitidine and antacid drugs have been identified; anticoagulants or platelet aggregation inhibitors - possible increase in bleeding time (increased risk of bleeding, INR control is required); beta-blockers and angiotensin-converting enzyme inhibitors may reduce their hypotensive effect; diuretics - reduces the diuretic effect and hypotensive effect; digoxin - reduces the renal clearance of digoxin. honolon antibiotics - increases the risk of developing seizures; other NSAIDs or glucocorticoids - increases the risk of gastrointestinal bleeding; methotrexate - the concentration of methotrexate in the serum increases; Selective serotonin reuptake inhibitors (eg, citalopram, fluoxetine, paroxetine, sertraline) increase the risk of gastrointestinal bleeding. lithium salts - may cause an increase in peak plasma lithium concentrations and thereby increase the known side effects of lithium; cyclosporine - the nephrotoxicity of cyclosporine increases. sulfonylurea derivatives - the hypoglycemic effect of the latter may be enhanced; alcohol, corticotropin, potassium supplements increase the risk of side effects from the gastrointestinal tract; cefamandole, cefoperazone, cefotetan, valproic acid increase the risk of bleeding.
Special instructions The risk of ulcerogenic effects of the drug can be reduced by the simultaneous administration of proton pump inhibitors and synthetic analogs of prostaglandins. If bleeding occurs in the gastrointestinal tract, the drug should be stopped immediately and appropriate emergency measures taken. It is especially necessary to carefully monitor the condition of those patients with gastrointestinal pathology who are receiving a course of treatment with XEFOCAM for the first time. Like other oxicams, XEFOCAM inhibits platelet aggregation and may therefore increase bleeding time. When using this drug, patients who require completely normal coagulation function (for example, patients undergoing surgery), who have coagulation disorders, or who are receiving medications that inhibit coagulation (including low-dose heparin) should be closely monitored when using this drug. , in order to promptly detect signs of bleeding. If signs of liver damage appear (itching, yellowing of the skin, nausea, vomiting, abdominal pain, dark urine, increased levels of liver transaminases), you should stop taking the drug and consult your doctor. The drug should not be used simultaneously with other NSAIDs. The drug can change the properties of platelets, but does not replace the preventive effect of acetylsalicylic acid in cardiovascular diseases. In patients with impaired renal function caused by large blood loss or severe dehydration, XEFOCAM, as an inhibitor of prostaglandin synthesis, can be prescribed only after hypovolemia and the associated risk of decreased renal perfusion have been eliminated. Like other NSAIDs, XEFOCAM can cause an increase in blood urea and creatinine concentrations, as well as water and sodium retention, peripheral edema, hypertension and other early signs of nephropathy. Long-term treatment of such patients with XEFOCAM can lead to the following consequences: glomerulonephritis, papillary necrosis and nephrotic syndrome with transition to acute renal failure. Patients with a pronounced decrease in renal function should not be prescribed XEFOCAM (see “Contraindications”). In elderly patients, as well as in patients suffering from arterial hypertension and/or obesity, it is necessary to control blood pressure levels. It is especially important to monitor renal function in elderly patients, as well as in patients: - simultaneously receiving diuretics; - concurrently receiving medications that can cause kidney damage. With long-term use of the drug XEFOCAM, it is necessary to periodically monitor hematological parameters, as well as renal and liver function. The use of the drug may adversely affect female fertility and is not recommended for women planning pregnancy. Patients using the drug must refrain from activities that require increased attention, rapid mental and motor reactions, and alcohol consumption.
Release form Film-coated tablets 4 mg and 8 mg. 10 tablets in a blister made of aluminum foil and PVC film. 1, 2, 3, 5 or 10 blisters with instructions for use are placed in a cardboard box.
Shelf life: 3 years. Do not use after the expiration date stated on the packaging.
Storage conditions
At a temperature not exceeding 25o C. Keep out of the reach of children.
Conditions for dispensing from pharmacies Dispensed by prescription
Name and address of the legal entity in whose name the registration certificate was issued
Takeda Austria GmbH, Austria St. Peter Strasse 25, A-4020 Linz, Austria (Takeda Austria GmbH, Austria St. Peter Strasse 25, A-4020 Linz, Austria)
Manufacturer
Takeda GmbH, Germany, Lehnitzstrasse 70-98, 16515, Oranienburg, Germany
Application
Pills
For all patients, the appropriate dosage regimen should be based on individual response to treatment.
Pain
The dose of lornoxicam is 8–16 mg/day, divided into 2–3 doses. The maximum recommended daily dose is 16 mg.
Osteoarthritis and rheumatoid arthritis
An initial dose of 12 mg of lornoxicam, divided into 2-3 doses, is recommended. The maintenance dose should not exceed 16 mg/day.
Xefocam film-coated tablets are taken orally with a sufficient amount of water.
Elderly patients (over 65 years of age), except for those with impaired liver or kidney function: no dose adjustment is required, but lornoxicam should be used with caution due to the likelihood of adverse reactions from the gastrointestinal tract.
Kidney failure. For patients with mild to moderate renal impairment, the maximum recommended daily dose is 12 mg, divided into 2-3 doses.
Liver failure. For patients with moderate hepatic impairment, the maximum recommended daily dose is 12 mg, divided into 2-3 doses (see SPECIAL INSTRUCTIONS).
Adverse reactions can be minimized by taking the drug at the lowest effective dose and for the shortest period necessary to control symptoms (see SPECIAL INSTRUCTIONS).
Injections
This dosage form of the drug is intended to initiate therapy and to quickly achieve an analgesic effect or in case it is impossible to use oral medications or suppositories. For all patients, the appropriate dosage regimen should be based on individual response to treatment.
For intravenous and intramuscular administration.
The recommended dose is 8 mg IV or IM. Some patients require an additional dose of 8 mg in the first 24 hours. The maximum daily dose is 16 mg.
The duration of intravenous administration of the solution should be at least 15 s, intramuscularly - at least 5 s. After preparing the solution, the needle should be replaced.
For intramuscular injection, a long needle is required, which ensures deep insertion. The medicine is intended for one-time use only. Prepare solution for injection immediately before use (the contents of 1 bottle (8 mg of lyophilisate) are dissolved with water for injection (2 ml)).
Elderly patients (over 65 years of age), except for those with impaired liver or kidney function, dosage adjustment is not required, but lornoxicam should be used with caution due to the likelihood of adverse reactions from the gastrointestinal tract.
Kidney failure. Patients with mild to moderate renal failure require a dose reduction.
Liver failure. Patients with moderate hepatic impairment require a dose reduction.
Adverse reactions can be minimized by taking the drug at the lowest effective dose and for the shortest period necessary to control symptoms (see SPECIAL INSTRUCTIONS).
Reconstituted solution: The chemical and physical stability of the reconstituted solution has been demonstrated for 24 hours at 2–8°C. From a microbiological point of view, the drug should be used immediately. In case of non-use immediately, the period and conditions of storage of the reconstituted solution before its use are the responsibility of the user.
Lornoxicam: a modern analgesic in the treatment of chronic pain syndrome
Correct and timely use of etiotropic or pathogenetic treatment can eliminate pain in most cases. Nevertheless, there are situations in which symptomatic pain therapy is indicated: with severe pain requiring immediate treatment, or in cases where the cause of pain cannot be eliminated [6]. Many acute and chronic diseases, medical interventions and injuries are associated with pain requiring the use of analgesics [1]. In clinical and outpatient practice, non-narcotic analgesics (painkillers) are widely used, which are separated from the group of non-steroidal anti-inflammatory drugs (NSAIDs) into a separate group, since the anti-inflammatory effect of most of these drugs is relatively weak. NSAIDs have an analgesic effect, which is combined with an antipyretic effect.
Non-narcotic analgesics include lornoxicam, ketorolac, paracetamol and metamizole sodium. The analgesic or analgesic effect of these drugs, like all NSAIDs, is due to the suppression of cyclooxygenase (COX) activity and a decrease in the production of prostaglandins E2 and F2α, which increase the sensitivity of nociceptors both during inflammation and tissue damage. A more pronounced analgesic than anti-inflammatory effect is possessed by those NSAIDs that, due to their chemical structure, are neutral, accumulate less in inflammatory tissue, penetrate the blood-brain barrier more quickly and suppress COX in the central nervous system, and also affect the thalamic centers of pain sensitivity (Table 1) [6, 7].
An NSAID with a pronounced analgesic effect is lornoxicam (Xefocam®, Takeda Pharmaceuticals LLC). Lornoxicam belongs to the oxicam class of NSAIDs and is a potent, balanced COX-1/COX-2 inhibitor. Unlike other members of the oxicam class, lornoxicam has a short half-life (approximately 4 hours), which reduces the risk of accumulation when taking multiple doses. Xefocam® has a powerful analgesic effect comparable to that of opioids. The drug is used in the treatment of acute pain from mild to moderately severe, to eliminate pain in renal colic, neurological diseases, injuries, in cancer patients, in the postoperative period, as well as in algodismenorrhea, lumbar ischialgia. In terms of analgesic activity, lornoxicam is superior to most NSAIDs, such as diclofenac, ketorolac and many others. To the same extent, unlike other analgesics, it also has a strong anti-inflammatory effect. Lornoxicam equally blocks COX-1 and COX-2, while in its ability to block COX it is superior to other drugs from the oxicam group. The anti-pain effect of the drug is due to both a violation of the generation of pain impulses and a weakening of the perception of pain. There is evidence that when administered parenterally, lornoxicam can increase the concentration of endogenous opioids, thereby activating the antinociceptive system. In addition, lornoxicam inhibits the release of oxygen free radicals from activated leukocytes. The drug does not affect opioid receptors and does not have a sedative or anxiolytic effect. In addition, it should be noted that in elderly patients with preserved liver and kidney function, no dose adjustment is required, which is also convenient for the physician prescribing the drug [6–21].
Ksefokam® is available in 3 forms (tablets Xefokam® and Xefokam® Rapid, Ksefokam® lyophilisate for injection), which is convenient from the point of view of choosing a therapeutic agent, since all therapeutic forms of the drug Xefokam® have relatively the same effect when switching from injection to oral form does not require dose adjustment. The presence of a parenteral form of lornoxicam is of particular value in the postoperative period. Xefocam® provides effective treatment of postoperative pain and demonstrates clinical effectiveness in abdominal, gynecological and orthopedic surgeries. When treating postoperative pain after abdominal surgery with Xefocam® for injection (iv), morphine consumption and the incidence of vomiting are reduced compared to tenoxicam. Xefocam® Rapid has faster absorption compared to standard Xefocam® tablets, which contributes to the rapid onset of the analgesic effect. Good tolerability due to safe pharmacokinetics (short half-life, inactive metabolites, absence of hepatotoxic action and accumulation of the drug in the body), as well as the “drug safety” of the drug, allow the drug to be widely and without complications used for the treatment of patients at risk: the elderly, obese, dysfunction of the liver (hepatitis) and kidneys, weakened patients, etc. [8–10, 19–25].
Lornoxicam is distinguished by its pronounced anti-inflammatory and analgesic effect and can be widely used in the practice of doctors of various specialties. A powerful balanced suppression of the activity of both COX isoenzymes, as well as stimulation of the production of endogenous dynorphin and endorphin, makes lornoxicam one of the most effective and safe modern analgesics for the relief of BS of any intensity and localization. Indications for the use of lornoxicam are short-term treatment of BS of various origins, symptomatic treatment of rheumatic diseases (rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, articular syndrome with exacerbation of gout, rheumatic soft tissue lesions) [6, 7, 19–21, 26].
The analgesic efficacy of lornoxicam (Xefocam®) was demonstrated in 3 meta-analyses, including 13 placebo-controlled studies evaluating various formulations and dosages ranging from 2 to 32 mg. Five studies assessed pain after surgical extraction of the 3rd molar. Five studies were conducted in patients with pain following major surgery (3 included general surgery, 1 included knee surgery, 1 included postpartum pain due to episiotomy). Three studies included patients with low back pain (1 with acute sciatica and 2 with chronic low back pain). For comparison, the meta-analysis included data obtained from the use of competitive drugs (acetylsalicylic acid, ibuprofen, naproxen, pethidine, diclofenac, tramadol, ketorolac and morphine). The results of the analysis showed that relatively small doses of Xefocam® have an effectiveness comparable to that of high doses of ketorolac and ibuprofen and medium doses of morphine, and have greater potency than low doses of acetylsalicylic acid, naproxen and ibuprofen. For example, the following ratios can be given: lornoxicam 4 mg tablets are equivalent in effectiveness to acetylsalicylic acid 650 mg and ibuprofen 200 mg, Xefocam® 8 mg tablets correspond to ibuprofen 400–800 mg and ketorolac 10 mg, for postoperative pain intravenous injections of lornoxicam 8– 16 mg is equivalent in effectiveness to morphine 20 mg, pethidine 100 mg and tramadol 50 mg. Moreover, unlike narcotic analgesics, lornoxicam does not have an effect on respiratory function, sedative, or psychomotor effects, and it is less likely to cause dyspepsia [26–31].
Of great interest is the ability of lornoxicam in chronic inflammatory arthropathy to quickly penetrate into the perivascular space, including synovial fluid, while other NSAIDs act mainly in the area of the inflamed synovium. Several foreign studies have shown the effectiveness of lornoxicam against BS in patients with OA. Thus, in a 4-week, double-blind, placebo-controlled, multicenter study involving 160 patients with hip and knee OA, it was shown that lornoxicam at a daily dose of 8 and 12 mg significantly reduced pain and improved joint function compared with placebo (5-5 mg each). Likert scale) – a dose of 12 mg was significantly more effective than 8 mg [17].
In a comparative study, the effectiveness of lornoxicam at a dose of 12 and 16 mg/day and diclofenac at a dose of 150 mg/day after 12 weeks. evaluated in 135 patients suffering from hip or knee OA. In all 3 comparison groups, after therapy, the functional status of patients with OA significantly improved (p<0.05 for paired assessment of effectiveness). Overall, 46% of patients across all treatment groups reported improvement in their disease, with pain relief rates (PAR) ranging from 42% to 48%. After 12 weeks When using lornoxicam at a dose of 16 mg/day, a significantly larger number of patients and doctors rated its effect as “good”, “very good” and “excellent” compared to the effect of diclofenac 150 mg/day and lornoxicam 12 mg/day [35].
Another comparative study (25-day multicenter) assessed the effectiveness of lornoxicam 16 mg/day and the selective COX-2 inhibitor rofecoxib 25 mg/day in 2520 patients with OA. During therapy with lornoxicam, there was a significant decrease in pain at night, during movement and at rest compared with the effect of therapy with a selective COX-2 inhibitor (p<0.01 in all cases). Although this was a non-randomized, double-blind clinical trial, the results are of interest due to the large number of participants. Overall, 40.9% of subjects rated the effectiveness of lornoxicam therapy as “excellent,” while in the comparison group only 20.1% of patients with OA reported an excellent result. Thus, lornoxicam therapy was significantly more effective against BS in patients with OA compared to therapy with a selective COX-2 inhibitor [18].
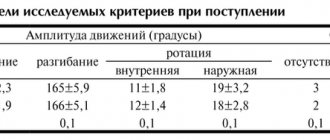
An overview of studies that evaluated the use of lornoxicam (Xefocam®) in RA or OA is presented in Table 2.
During a 3-week double-blind multicenter study involving 316 patients, the effectiveness of standard Xefocam® tablets at a daily dose of 12 mg and diclofenac at a daily dose of 150 mg was compared in RA. For all clinical parameters assessed (Ritchey joint index, duration and severity of morning stiffness, severity of pain and number of painful joints), there was a significant improvement in indicators compared to baseline indicators from the first week of treatment onwards (in all groups p<0.01 relative to baseline indicators on the 3rd week of the study). There were no significant differences between the 2 drugs. According to doctors, therapy with Xefocam® was assessed well and satisfactorily in 75% of patients compared to 69% of those who took diclofenac (Fig. 1) [15].
In a 12-week, prospective, randomized, multicenter, double-blind, parallel-group study in patients with RA, the effectiveness of the following drugs was compared: standard Xefocam® 4 mg tablets three times a day, standard Xefocam® tablets 8 mg twice a day, and 500 mg naproxen 2 rubles/day After 12 weeks. patients could continue treatment over the next 40 weeks, while they took Xefocam® either at the same dose or according to one of two therapeutic regimens for using the drug Xefocam®. The primary objective of the study was to examine changes in grip strength after 12 weeks. treatment. On average, changes in the strength of morning stiffness in the group of patients taking standard Xefocam® tablets at a dose of 4 mg 3 times a day and standard Xefocam® tablets at a dose of 8 mg 2 times a day were similar to those taking naproxen in the initial 12-12 days. week study period, as well as in those taking standard Xefocam® tablets at a dose of 4 mg 3 times / day and 8 mg 2 times / day during the subsequent 40-week study period. Improvements in mean scores for all parameters, including Ritchie joint index, subjective pain rating, functional status, disease progression, and PAR, were similar across treatment regimens. Lornoxicam has proven its effectiveness in reducing pain and inflammation in RA, which was confirmed by the results of assessing all significant clinical parameters [16].
A review of the clinical safety data for lornoxicam shows that its safety profile is comparable to that of other traditional NSAIDs. In addition, it should be noted that in the pooled analysis, Xefocam® was significantly better tolerated than other comparator drugs. Although Xefocam® did not increase the risk of side effects in patients over 65 years of age, the drug should nevertheless be prescribed with caution, taking into account the fact that gastrointestinal side effects are particularly difficult to tolerate in this age group. The favorable safety profile of Xefocam® is a consequence of the fact that the drug is a balanced COX-1/COX-2 inhibitor and has a relatively short half-life compared to that of other NSAIDs [36].
Conclusion
Analgesics play an important role in the treatment of chronic BS. Choosing an effective and safe drug is an urgent task for specialists. Lornoxicam (Xefocam®) is an NSAID with powerful analgesic activity. The analgesic effect of lornoxicam exceeds that of many other NSAIDs and is comparable in strength to narcotic analgesics. The safety of Xefocam® has been clinically proven and confirmed in studies.
Lornoxicam is a modern analgesic that provides sufficient effect for pain of any intensity, and its widespread use both in outpatient clinics, emergency rooms, and in multidisciplinary hospitals is justified from the standpoint of evidence-based medicine. The availability of various dosage forms of the drug Xefokam® - tablets Xefokam® and Xefokam® Rapid, as well as Xefokam® for injections - determine the convenience for both the specialist and the patient.
Contraindications
- Hypersensitivity to lornoxicam or other components of the drug; thrombocytopenia; hypersensitivity (symptoms similar to those of asthma, rhinitis, angioedema or urticaria) to other NSAIDs, including acetylsalicylic acid; severe form of heart failure; gastrointestinal bleeding, cerebrovascular or other bleeding; a history of gastrointestinal bleeding or ulcer perforation associated with previous NSAID therapy; active recurrent peptic ulcer/bleeding or history of recurrent peptic ulcer/bleeding (2 or more separate proven episodes of ulceration or bleeding); severe form of liver failure; severe form of renal failure (plasma creatinine level 700 µmol/l); iii trimester of pregnancy (see use during pregnancy and lactation).
Side effects
Most often, adverse reactions to NSAIDs were associated with the gastrointestinal tract. When using NSAIDs, peptic ulcers, perforation or gastrointestinal bleeding may occur, which is sometimes fatal, especially in the elderly (see special instructions). nausea, vomiting, diarrhea, flatulence, constipation, dyspepsia, abdominal pain, melena, hematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn's disease have been reported with NSAID treatment. Gastritis occurred less frequently.
It is estimated that ≈20% of patients treated with lornoxicam may experience adverse events. The most common side effects of lornoxicam are nausea, dyspepsia, indigestion, abdominal pain, vomiting, and diarrhea. These symptoms overall were observed in 10% of patients in the study. Edema, hypertension and heart failure have been reported with NSAID treatment.
Clinical studies and epidemiological data indicate that the use of some NSAIDs, especially in high doses and over long periods of time, may be associated with an increased risk of arterial thrombotic events such as myocardial infarction or stroke (see WARNINGS).
Serious infectious complications of the skin and soft tissues have been reported exclusively during the course of chickenpox.
Adverse effects according to the frequency of occurrence are classified into the following categories: very often (≥1/10); often (≥1/100, 1/10); uncommon (≥1/1000, 1/100); rare (≥1/10,000, 1/1000); very rare (1/10,000), unknown (frequency cannot be estimated from available data).
Infections and infestations: rarely - pharyngitis.
From the blood and lymphatic system: rarely - anemia, thrombocytopenia, leukopenia, increased duration of bleeding; very rarely - ecchymosis. NSAIDs can cause class-specific, potentially severe hematological disorders, such as neutropenia, agranulocytosis, aplastic anemia, hemolytic anemia.
From the immune system: rarely - hypersensitivity reactions, anaphylactoid reactions and anaphylaxis.
Metabolic disorders: uncommon - loss of appetite, changes in body weight.
Mental disorders: infrequently - insomnia, depression; rarely - anxiety, nervousness, excitement.
From the nervous system: often - mild and transient headache, dizziness; rarely - drowsiness, paresthesia, taste disturbances (dysgeusia), tremor, migraine; very rarely - aseptic meningitis in patients with systemic lupus erythematosus and mixed connective tissue disease (see SPECIAL INSTRUCTIONS).
From the organ of vision: infrequently - conjunctivitis; rarely - visual impairment.
On the part of the organ of hearing and balance: infrequently - vertigo, tinnitus.
From the cardiovascular system: infrequently - palpitations, tachycardia, edema, heart failure, facial flushing; rarely - hypertension, hot flashes, hemorrhages, hematomas.
From the respiratory system: infrequently - rhinitis; rarely - dyspnea, cough, bronchospasm.
From the digestive system: often - nausea, abdominal pain, dyspepsia, diarrhea, vomiting; uncommon - constipation, flatulence, belching, dry mouth, gastritis, stomach ulcer, abdominal pain, abdominal pain in the upper abdomen, duodenal ulcer, ulcers of the oral mucosa; rarely - melena, vomiting with blood, stomatitis, esophagitis, gastroesophageal reflux, dysphagia, aphthous stomatitis, glossitis, ulcer perforation, gastrointestinal bleeding.
From the liver and biliary tract: infrequently - increased levels of liver enzymes (ALAT, AST); very rarely - toxic effects on the liver, which may result in the development of liver failure, hepatitis, jaundice, cholestasis.
From the skin and subcutaneous tissues: infrequently - rash, itching, increased sweating, erythematous rash, urticaria, angioedema, alopecia; rarely - dermatitis, eczema, purpura; very rarely - swelling and bullous reactions, such as erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
From the musculoskeletal system and connective tissue: infrequently - arthralgia; rarely - a feeling of pain in the bones, muscle spasms, myalgia.
From the kidneys and urinary tract: rarely - nocturia, difficulty urinating, increased levels of urea nitrogen and creatinine in the blood; very rarely, lornoxicam may cause acute renal failure in persons with kidney disease, which is dependent on renal prostaglandins, which have an important role in maintaining renal blood flow (see PRECAUTIONS). Nephrotoxicity in various forms, including nephritis and nephrotic syndrome, is a class-specific effect of NSAIDs.
General disorders: infrequently - malaise, swelling of the face; rarely - asthenia.
Xefocam lyophilisate (solution)
In each particular category, side effects are grouped by system-organ class and presented in descending order of frequency: very often (≥ 1/10); often (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to < 1/100); rare (≥ 1/10,000 to < 1/1000); very rare (< 1/10,000), unknown (cannot be estimated based on available data).
Infectious and parasitic diseases
Rarely: pharyngitis.
Blood and lymphatic system disorders
Rarely: anemia, thrombocytopenia, leukopenia, increased bleeding time.
Very rare: ecchymosis. NSAIDs have been reported to cause potentially severe hematological disorders, such as neutropenia, agranulocytosis, aplastic anemia and hemolytic anemia (class-specific effects).
Immune system disorders
Rarely: hypersensitivity, anaphylactoid and anaphylactic reactions.
Metabolic and nutritional disorders
Uncommon: anorexia, weight change.
Mental disorders
Uncommon: sleep disturbance, depression.
Rarely: confusion, nervousness, agitation.
Nervous system disorders
Often: short-term headaches of low intensity, dizziness.
Rarely: somnolence, paresthesia, taste disturbance, tremor, migraine.
Very rare: aseptic meningitis in patients with SLE and mixed connective tissue diseases.
Visual disorders
Uncommon: conjunctivitis.
Rarely: visual disturbances.
Hearing and labyrinth disorders
Uncommon: dizziness, tinnitus.
Heart disorders
Uncommon: palpitations, tachycardia, edema, heart failure.
Vascular disorders
Uncommon: flushing of the face, swelling.
Rarely: arterial hypertension, bleeding, hematoma.
Respiratory, thoracic and mediastinal disorders
Uncommon: rhinitis.
Rarely: dyspnea, cough, bronchospasm.
Gastrointestinal disorders
Common: nausea, abdominal pain, dyspepsia, diarrhea, vomiting.
Uncommon: constipation, flatulence, belching, dry mouth, gastritis, gastric ulcer, epigastric pain, duodenal ulcer, oral ulceration.
Rarely: melena, hematemesis, stomatitis, esophagitis, gastroesophageal reflux, dysphagia, aphthous stomatitis, glossitis, perforated peptic ulcer, gastrointestinal bleeding.
Disorders of the liver and biliary tract
Uncommon: increased liver function tests, alanine aminotransferase (ALT) or aspartate aminotransferase (AST).
Rarely: liver dysfunction.
Very rare: damage to hepatocytes. Hepatotoxicity, which can lead to liver failure, hepatitis, jaundice and cholestasis.
Skin and subcutaneous tissue disorders
Uncommon: rash, itching, sweating, erythematous rash, urticaria, Quincke's edema, alopecia.
Rarely: dermatitis and eczema, purpura.
Very rare: edema, bullous reactions, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Musculoskeletal and connective tissue disorders
Uncommon: arthralgia.
Rarely: bone pain, muscle spasms, myalgia.
Renal and urinary tract disorders
Rarely: nocturia, urinary disorders, increased levels of urea and creatinine in the blood.
Very rare: In patients with pre-existing renal impairment who require renal prostaglandins to maintain renal blood flow, lornoxicam may precipitate acute renal failure. Nephrotoxicity in various forms, including nephritis and nephrotic syndrome, is a class-specific effect of NSAIDs.
General and administration site disorders
Uncommon: malaise, facial swelling.
Rarely: asthenia.
special instructions
For the following disorders, the drug can be prescribed only after a thorough assessment of the ratio of the expected benefit of therapy/possible risk.
Lornoxicam should be used with caution in patients with mild renal impairment (serum creatinine levels 150–300 μmol/L) and moderate renal impairment (serum creatinine levels 300–700 μmol/L) due to the important role of prostaglandins in maintaining renal blood flow. If renal function deteriorates, treatment with the drug should be discontinued.
People who have had major surgery, have heart failure, or are taking diuretics or medications that can cause kidney damage should have their kidney function closely monitored.
In patients with bleeding disorders, a thorough clinical examination and evaluation of laboratory parameters (eg activated partial thrombin time) are recommended.
Persons with liver failure (for example, cirrhosis of the liver) after using the drug at a dose of 12-16 mg / day are recommended to regularly conduct laboratory tests due to the possibility of accumulation of lornoxicam in the body (increased AUC). But no deviations in pharmacokinetic parameters were detected in patients with liver failure compared to healthy volunteers.
During long-term treatment (3 months), it is recommended to assess blood status (hemoglobin determination), kidney function (creatinine determination) and liver enzymes.
Elderly people (over 65 years of age) are advised to monitor kidney and liver function and use the drug with caution after surgery.
The combined use of lornoxicam with other NSAIDs, including selective COX-2 inhibitors, should be avoided.
Adverse reactions can be minimized by taking the lowest effective dose of the drug for the short period necessary to control symptoms of the disease.
When using any NSAID at any time during treatment, gastrointestinal bleeding, ulceration or perforation may occur (with or without warning symptoms or a history of serious gastrointestinal disorders), which can be fatal.
The risk of gastrointestinal bleeding, ulceration or perforation increases with increasing doses of NSAIDs in patients with a history of ulcers, especially those complicated by bleeding or perforation (see CONTRAINDICATIONS), as well as in the elderly. These groups of patients should be especially careful when starting treatment with the drug at the lowest therapeutic doses.
NSAIDs should be used with caution to treat the above groups of patients and persons concurrently taking acetylsalicylic acid in low doses or other drugs that increase the risk of gastrointestinal complications (see INTERACTIONS). For patients requiring such combination therapy, treatment can be carried out while taking protective agents (eg misoprostol or proton pump inhibitors). Clinical follow-up at regular intervals is recommended.
Patients with a history of GI toxicity, especially the elderly, should report any unusual abdominal symptoms (especially GI bleeding) during the initial stages of treatment. Use extreme caution in patients concomitantly using medications that may increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors, or antithrombotic drugs such as acetylsalicylic acid (see INTERACTIONS).
If bleeding or gastrointestinal ulceration occurs in patients taking lornoxicam, treatment should be discontinued.
NSAIDs should be used with caution in patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), as their condition may worsen. In elderly people, the incidence of adverse reactions when using NSAIDs increases, in particular gastrointestinal bleeding and perforation, which can be fatal (see CONTRAINDICATIONS). The drug should be used with caution in patients with hypertension and/or a history of heart failure, since edema and fluid retention in the body may occur as a result of taking NSAIDs.
Patients with a history of hypertension and/or congestive heart failure of mild to moderate severity should be monitored, since NSAID therapy may be accompanied by phenomena such as fluid retention and edema. There are clinical studies and epidemiological data that suggest that the use of some NSAIDs (especially long-term therapy and at high doses) may be associated with a small increased risk of arterial thrombotic events (eg myocardial infarction or stroke). There is insufficient data to exclude such a risk with lornoxicam.
In patients with uncontrolled hypertension, chronic heart failure, coronary artery disease, peripheral arterial disease and/or cerebrovascular disease, lornoxicam should be prescribed only after careful evaluation of the indications. Evaluation is also required before initiating long-term treatment in individuals with cardiovascular risk factors (eg, hypertension, hyperlipidemia, diabetes mellitus, smoking). Combination treatment with NSAIDs and heparin increases the risk of spinal/epidural hematoma during spinal or epidural anesthesia (see INTERACTIONS).
Very rarely, skin reactions occur with the use of NSAIDs, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis, sometimes some of them are fatal (see SIDE EFFECTS). The risk of developing such reactions is highest at the beginning of treatment: in most cases, such reactions occur in the 1st month of taking the drug. Lornoxicam should be discontinued at the first sign of skin rash, mucosal lesions, and other manifestations of hypersensitivity.
Use with caution in persons with asthma or a history of this disease, since NSAIDs provoke bronchospasm in these patients.
Patients with systemic lupus erythematosus and mixed connective tissue disease may have an increased risk of developing aseptic meningitis.
Lornoxicam inhibits platelet aggregation, increasing blood clotting time.
The drug should be prescribed with caution to patients with a tendency to bleeding.
Concomitant treatment with NSAIDs and tacrolimus may increase the risk of nephrotoxicity due to decreased renal prostacyclin synthesis. Renal function must be carefully monitored during this combination therapy.
Like other NSAIDs, lornoxicam may cause episodic increases in transaminases, serum bilirubin, as well as increases in blood urea and creatinine concentrations, as well as other laboratory abnormalities. If the deviations in laboratory parameters are significant and continue for a long time, treatment must be stopped and the necessary research carried out.
The drug contains lactose. Patients with rare hereditary galactose intolerance, lactase deficiency or glucose-galactose malabsorption should not use the drug.
Lornoxicam, like other drugs that suppress COX/prostaglandin synthesis, can reduce fertility and is therefore not recommended for use in women planning pregnancy. Women who have problems becoming pregnant or who are undergoing evaluation for infertility should stop taking lornoxicam.
In the presence of chickenpox, in exceptional cases, severe infectious lesions of the skin and soft tissues may develop. By this time, the influence of NSAIDs on the worsening of these infectious diseases cannot be ruled out. It is recommended to avoid the use of lornoxicam if you have chickenpox.
Use during pregnancy or breastfeeding. Pregnancy. Lornoxicam is contraindicated in the third trimester of pregnancy. There are no clinical data on the use of lornoxicam in the first and second trimesters of pregnancy and during childbirth, so the drug is not recommended for use during this period.
There is insufficient data regarding the use of lornoxicam in pregnant women. Animal studies have shown reproductive toxicity.
Suppression of prostaglandin synthesis may adversely affect pregnancy and/or embryonic/fetal development. Data from epidemiological studies indicate an increased risk of miscarriage, as well as the development of heart defects when using prostaglandin synthesis inhibitors in early pregnancy. The risk increases with increasing dose and duration of therapy. In animals, the use of prostaglandin synthesis inhibitors leads to an increase in pre- and post-implantation fetal death and embryofetal mortality. Prostaglandin synthesis inhibitors should not be used in the first and second trimesters of pregnancy. Use is possible only when absolutely necessary.
In the third trimester of pregnancy, when using any prostaglandin synthesis inhibitors, the following effects on the fetus are possible:
- cardiopulmonary toxicity (premature closure of the ductus arteriosus and pulmonary hypertension);
- impaired renal function, which can progress to renal failure with the manifestation of oligohydroamnion.
The pregnant woman and the fetus at the end of pregnancy may be exposed to the following effects from the use of prostaglandin synthesis inhibitors:
- possible increase in the duration of bleeding;
- suppression of the contractile function of the uterus, which can lead to a delay or increase in the duration of labor.
Thus, the use of lornoxicam is contraindicated in the third trimester of pregnancy (see CONTRAINDICATIONS).
Breastfeeding period. There is no data on the excretion of lornoxicam into human breast milk. Relatively high concentrations of lornoxicam are excreted into the milk of lactating rats. Therefore, lornoxicam should not be used during breastfeeding.
Children. Lornoxicam is not recommended for use in children under 18 years of age due to the lack of clinical data on the effectiveness and safety of the drug.
The ability to influence reaction speed when driving vehicles or working with other mechanisms. If dizziness and/or drowsiness occurs as a result of taking lornoxicam, you should not drive vehicles or operate other machinery.
Lornoxicam
Lornoxicam inhibits platelet aggregation and prolongs bleeding time, so the drug should be used with caution if there is an increased tendency to bleeding.
For the following disorders, Lornoxicam should be used only after a careful assessment of the expected benefits of therapy and the possible risks in:
- patients with impaired renal function: from mild (serum creatinine 150-300 µmol/l) to moderate (serum creatinine 300-700 µmol/l), since the maintenance of renal blood flow depends on the level of renal prostaglandins. The use of Lornoxicam should be discontinued if renal function deteriorates during treatment.
Renal function should be monitored in patients:
- who have undergone extensive surgery,
- with heart failure,
- receiving concomitant treatment with diuretics or drugs with proven or suspected nephrotoxicity;
- patients with a disorder of the blood coagulation system: careful clinical observation and assessment of laboratory parameters, for example, activated partial thrombin time, are recommended;
- patients with impaired liver function (liver cirrhosis): it is recommended to carry out regular clinical observation and assessment of laboratory parameters, since when treated with lornoxicam at a daily dose of 12-16 mg, accumulation of the drug is possible;
- patients over 65 years of age: monitoring of liver and kidney function is recommended. Use with caution in elderly patients in the postoperative period.
Concomitant use with NSAIDs
Concomitant use with other NSAIDs, including selective cyclooxygenase type 2 (COX-2) inhibitors, should be avoided.
Minimizing unwanted effects
Undesirable effects can be minimized by using the lowest effective dose of the drug for the shortest period of time sufficient to control symptoms.
Gastrointestinal bleeding, ulcer, gastrointestinal perforation
Gastrointestinal bleeding, ulcers, and perforation can occur with the use of any NSAIDs and can be fatal. Warning symptoms or serious gastrointestinal pathology may not be present in the patient's medical history.
The risk of bleeding, ulceration or perforation of the gastrointestinal tract increases with increasing doses of NSAIDs, in patients with a history of gastrointestinal ulceration, especially complicated by bleeding or perforation, and in elderly patients. Such patients should begin treatment with the lowest possible dose. In these patients, as well as patients receiving low-dose acetylsalicylic acid therapy or other drugs that may increase gastrointestinal risk, combination therapy with gastroprotective agents (eg, misoprostol or proton pump inhibitors) should be considered. It is recommended that such patients be regularly assessed.
Patients with a history of GI side effects, especially in the elderly, should be instructed to report any unusual GI symptoms (especially GI bleeding), especially early in treatment.
Lornoxicam should be used with caution while taking medicinal products that may increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (eg, warfarin), selective serotonin reuptake inhibitors, and antiplatelet drugs (eg, acetylsalicylic acid).
If bleeding or ulceration of the gastrointestinal tract develops while taking lornoxicam, treatment should be discontinued.
NSAIDs should be used with caution in patients with a history of gastrointestinal pathology (ulcerative colitis, Crohn's disease), as the patient's condition may worsen.
Elderly patients
Elderly patients have an increased incidence of adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation, which can be fatal.
Cardiovascular and cerebrovascular diseases
Patients with a history of or current hypertension and/or mild to moderate congestive heart failure require appropriate monitoring and counseling as cases of fluid retention and edema have been reported with the use of NSAIDs.
Data from clinical and epidemiological studies indicate that some NSAIDs, especially at high doses and with long-term use, may increase the risk of arterial thromboembolic events (eg, myocardial infarction or stroke). There is insufficient data to rule out such a risk for lornoxicam.
Lornoxicam should be administered with extreme caution to patients with uncontrolled hypertension, congestive heart failure, established coronary artery disease, peripheral arterial disease and/or cerebrovascular disease. The same caution is necessary with long-term administration of the drug to patients who have risk factors for cardiovascular diseases (for example, arterial hypertension, hyperlipidemia, diabetes mellitus, smoking).
With the simultaneous use of NSAIDs and heparin during spinal and epidural anesthesia, the risk of developing hematoma increases.
Skin diseases
In very rare cases, severe skin reactions, some fatal, have been reported in association with NSAID use, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis. The risk of such reactions is highest at the beginning of therapy - the development of the reaction is observed in the first month of treatment in most cases. The use of lornoxicam should be discontinued at the first appearance of skin rash, mucous membrane eruptions or any other signs of hypersensitivity.
Respiratory diseases
WITH
the drug should be used with caution in patients with active bronchial asthma or a history of asthma, since it is known that NSAIDs can provoke bronchospasm in such patients.
Systemic lupus erythematosus and mixed connective tissue diseases
Caution should be exercised when using lornoxicam in patients with SLE and mixed connective tissue diseases, as they may have an increased risk of aseptic meningitis.
Nephrotoxicity
Concomitant use of NSAIDs and tacrolimus may increase the risk of nephrotoxicity due to inhibition of renal prostacyclin synthesis. During combination therapy with these drugs, renal function must be carefully monitored.
Laboratory indicators
As with most NSAIDs, increases in serum aminotransferases, serum bilirubin levels, or other biochemical indicators of liver function, as well as increases in serum creatinine and urea levels, and other laboratory abnormalities have been reported. If any of these abnormalities are significant or persist for a long time, the use of lornoxicam should be discontinued and appropriate studies should be ordered.
Fertility
Lornoxicam, like any drug that inhibits cyclooxygenase and prostaglandin synthesis, may impair fertility and is not recommended for women planning pregnancy. If a woman is unable to become pregnant or is being evaluated for infertility, lornoxicam should be discontinued.
Chicken pox
It is recommended to avoid the use of lornoxicam in patients with serious skin and soft tissue infections caused by varicella zoster virus. To date, a contributing role of NSAIDs in exacerbating these infections cannot be ruled out. Thus, it is advisable to avoid the use of lornoxicam for chickenpox.
Interactions
Concomitant use of lornoxicam and the following drugs:
- cimetidine: increased plasma concentrations of lornoxicam (no interactions between lornoxicam and ranitidine or lornoxicam and antacids have been identified);
- Anticoagulants: NSAIDs may increase the severity of the effects of anticoagulants (for example, warfarin - see SPECIAL INSTRUCTIONS). MNI should be closely monitored;
- phenprocoumon: the effectiveness of treatment with phenprocoumon decreases;
- heparin: NSAIDs increase the risk of spinal/epidural hematoma when used concomitantly with heparin during spinal or epidural anesthesia (see WARNINGS);
- ACE inhibitors: may reduce the severity of the effect of ACE inhibitors;
- diuretics: weakening of the diuretic and hypotensive effect of loop, thiazide and potassium-sparing diuretics;
- β-adrenergic receptor blockers: weakening of the hypotensive effect;
- Angiotensin II receptor blockers: decreased hypotensive effect;
- Digoxin: decreased renal clearance of digoxin;
- corticosteroids: increased risk of gastrointestinal ulcers or bleeding (see WARNINGS);
- antibacterial agents of the quinolone group: increases the risk of seizures;
- antiplatelet drugs: increases the risk of gastrointestinal bleeding (see SPECIAL INSTRUCTIONS);
- other NSAIDs: increases the risk of gastrointestinal bleeding;
- methotrexate: an increase in the concentration of methotrexate in the blood plasma, which leads to an increase in its toxicity. Close monitoring is necessary during concurrent use;
- SSRIs: increases the risk of gastrointestinal bleeding (see SPECIAL INSTRUCTIONS);
- Lithium preparations: NSAIDs reduce the renal clearance of lithium, so serum lithium concentrations may exceed the toxicity threshold. It is necessary to monitor the level of lithium in the blood serum, especially at the beginning of treatment, when adjusting the dose and stopping treatment;
- cyclosporine: increased concentration of cyclosporine in the blood serum. Increased nephrotoxicity of cyclosporine is possible due to effects mediated by renal prostaglandins. During combination therapy, it is necessary to monitor renal function;
- sulfonylurea derivatives (for example glibenclamide): the risk of hypoglycemia will increase;
- known inducers and inhibitors of CYP 2C9 isoenzymes: lornoxicam (like other NSAIDs dependent on cytochrome P450 2C9 (CYP 2C9 isoenzyme)) interacts with known inducers and inhibitors of CYP 2C9 isoenzymes (see Biotransformation);
- tacrolimus: increased risk of nephrotoxicity due to decreased renal prostacyclin synthesis. During combination therapy, it is necessary to monitor renal function (see SPECIAL INSTRUCTIONS);
- pemetrexed: NSAIDs may decrease the renal clearance of pemetrexed, resulting in increased renal and gastrointestinal toxicity and myelosuppression.
Since food slows down the absorption of lornoxicam, Xefocam tablets should not be taken with food if a rapid onset of effective action (pain relief) is required.
Eating reduces absorption by ≈20% and increases Tmax.
Incompatibility. The drug in the form of an injection solution should not be mixed with other drugs, except those specified in the instructions for use.
Overdose
Currently, there is no data regarding overdose that would allow us to determine its consequences or suggest specific treatment. As a result of an overdose of lornoxicam, the following symptoms may occur: nausea, vomiting, cerebral symptoms (dizziness, blurred vision). in severe cases: ataxia, with transition to coma and convulsions; liver and kidney damage; Possible blood clotting disorder. in case of real or suspected overdose, use of the drug should be discontinued. Thanks to the short half-life, lornoxicam is quickly eliminated from the body. dialysis is ineffective. There is currently no specific antidote. The usual emergency measures, including gastric lavage, should be carried out. Based on general principles, only the use of activated carbon when taken immediately after an overdose of lornoxicam can lead to a decrease in absorption of the drug. For the treatment of gastrointestinal disorders, you can, for example, use a prostaglandin analogue or ranitidine.
Note!
Description of the drug Xefocam por. d/r-well d/in. 8mg vial. No. 5 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.