Calcium and potassium preparations are a group of medicines that contain salts of macroelements - calcium and potassium, respectively.
Macro- and microelements are a collective (generalized) name for mineral substances - food components that, together with vitamins, are involved in maintaining normal human life.
If minerals are needed by the body in relatively large quantities, they are called macroelements. Macroelements include magnesium, calcium, potassium, sodium, phosphorus, chlorine and sulfur.
The human body requires minerals called microelements in much smaller quantities. Microelements include zinc, iodine, selenium, iron, manganese, copper, etc.
The most important macroelements that regulate the normal functioning of the human body include calcium and potassium.
Calcium and potassium are active participants in many biochemical processes in the body, significantly influencing metabolism.
By depositing (accumulating) in the bones, calcium gives them strength and elasticity and prevents the occurrence of fractures. In addition, the importance of calcium is in the implementation of muscle contractions. Calcium is involved in the conduction of nerve impulses and the regulation of excitation processes in the brain. In addition, calcium ions play a key role in blood clotting and the formation of blood clots (thrombi), which is a key factor in stopping bleeding.
Potassium maintains osmotic pressure in cells and also regulates the acid-base balance of the body. Potassium is also involved in the conduction of excitation along nerve fibers, as well as in the transmission of impulses through the conduction system of the heart, and affects neuromuscular transmission.
With a balanced diet, the human body receives sufficient amounts of calcium and potassium from food. However, under certain conditions (for example, poor nutrition, significant physical activity, various pathological conditions, taking certain medications), there is a deficiency of these macroelements. In this case, drugs containing calcium or potassium are used.
IMPORTANCE OF POTASSIUM FOR CVS
Let's take a closer look at the effect of potassium on heart function. The electrical activity of myocardial cells depends on transmembrane ionic gradients, as well as on time- and voltage-dependent disturbances in the conduction of ionic currents. Electrolyte abnormalities can cause or facilitate the development of clinically significant arrhythmias even in normal cardiac tissue by modulating ion conduction through specific myocardial ion channels. The Na+ current into the cell upon activation of Na+ channels forms a phase of rapid depolarization in cardiomyocytes. As depolarization increases, permeability to Na+ decreases due to inactivation of Na+ channels, but channels for incoming Ca++ currents, which are necessary for the formation of the plateau phase, open. Subsequent activation of potassium channels leads to repolarization of the cardiomyocyte membrane to the level of the PP. Potassium, especially extracellular potassium, is the most important factor determining membrane PP. The electrophysiological effects of potassium depend not only on its extracellular concentration, but also on the direction (hypo- or hyperkalemia) and the rate of its change. Potassium ion channels are of primary importance for the regulation of the transmembrane potassium gradient. Potassium channels are transmembrane proteins that selectively allow potassium ions to pass through: potassium moves under the influence of an electrochemical gradient at a rate of 106 to 108 ions per second [1]. There are voltage-gated potassium channels and numerous channels that open for potassium ions or are blocked by various substances - ligands of the corresponding receptors. Such channels maintain the background conductivity of membranes for potassium ions and form the PP of excitable and non-excitable cells*. Hypokalemia (<3 mmol/L) reduces membrane permeability to potassium [24]. Thus, the conductance for the incoming (anomalous) potassium rectifying current is proportional to the square root of the extracellular potassium concentration [20, 21]. The dependence of the activation of the delayed (outgoing) rectifying current on the extracellular potassium concentration helps to understand why the AP duration is shorter at high potassium concentrations and longer at low potassium concentrations [31]. But the effects of potassium on the membrane PP are also modulated by the simultaneously created Ca++ concentrations. Their interaction is such that increased Ca++ levels reduce the depolarizing effect caused by increased potassium levels. In turn, low Ca++ levels attenuate the depolarization caused by hypokalemia.
Factors stimulating the transmembrane movement of potassium:
- from the cell to the extracellular space:
– acidosis; – stimulation of α-adrenergic receptors; – digitalis preparations;
- from the extracellular space into the cell:
– alkalosis; – stimulation of β2-adrenergic receptors; – insulin.
Damage to membrane phospholipids in the processes of lipid peroxidation (LPO) also leads to disruption of the barrier function of the membrane and increased potassium loss by the cell. Activation of LPO occurs in pathological conditions such as dystrophy, inflammation, myocardial ischemia, etc.
What is hyperkalemia?
Hyperkalemia is a condition in which serum potassium levels exceed 5 mmol/L. Hyperkalemia occurs in 1-10% of patients admitted to hospitals.
Hyperkalemia is a condition in which serum potassium levels exceed 5 mmol/L.
Hyperkalemia occurs in 1-10% of patients admitted to hospitals. The number of patients with hyperkalemia has increased in recent years due to an increase in the number of patients taking medications that affect the renin-angiotensin-aldosterone system (RAAS). Potassium inside the human body
Potassium is the most important electrolyte in the human body.
It plays a key role in the conduction of nerve impulses and muscle contraction. 98% of potassium is concentrated in the intracellular fluid; the potassium concentration here reaches 140 mmol/l. Only 2% of potassium is found outside the cells, the concentration here is 3.8-5.0 mmol/l. The role of potassium in the body
Potassium is the main intracellular cation (a positively charged ion), in contrast to sodium, the main extracellular cation.
Functionally, potassium and sodium are related to each other:
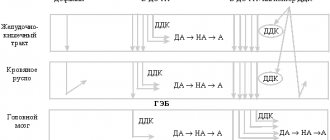
- The creation of membrane potential, important for muscle contraction (skeletal and cardiac muscle), is ensured by maintaining a high concentration of sodium outside the cell and potassium inside the cell (sodium-potassium pump, see Fig. 1).
- Maintaining acid-base balance, osmotic balance, water balance
- Activation of many enzymes
Mechanisms of regulation of potassium metabolism
To maintain normal potassium balance (transport between intra- and extracellular fluid), a coordinated interaction of all regulatory mechanisms is required. The main mechanism for regulating potassium levels is its excretion by the kidneys . This mechanism is controlled by the adrenal hormone aldosterone . The presence of this mechanism ensures that, despite the high potassium content in food (from 40 mmol to 200 mmol), its level in the blood serum will be maintained at a constant level. Dysregulation of potassium levels, and, as a result, an increase in potassium levels in the blood, can alter the excitability of membranes. This means that the function of nerves, muscles, and heart will be impaired.
Fig. 1 Scheme of regulation of transmembrane potassium transport Potassium concentration inside cells is maintained by active transport of potassium using Na-K-ATPase and passively due to a concentration gradient. The rate of passive movement depends on the permeability of potassium channels in the cell membrane. Insulin and beta-2 adrenergic agonists, through cAMP, promote the uptake of potassium into cells by stimulating Na-K-ATPase. With insulin deficiency and the action of beta-2 blockers, the release of potassium from the cells increases, which leads to hyperkalemia. Acidosis, hyperosmolarity, and cell lysis also cause potassium leakage and an increase in potassium levels in the blood. In the diagram: ECF=extracelluar fluid (intracellular fluid); ICF=intracellular fluid Aldosterone is a mineralocorticoid hormone synthesized in the adrenal cortex from cholesterol.
Under the influence of aldosterone in the kidneys, tubular reabsorption (that is, reverse absorption from primary urine) of sodium ions increases: aldosterone stimulates the transition of sodium into the cells, and potassium - outward (into the intercellular space, that is, potassium then passes into the urine, released from the body) - see .fig.2. Aldosterone also increases the secretion of potassium and hydrogen ions by the kidneys. Thus, the body's sodium and extracellular fluid levels (the body retains water) increase. Aldosterone levels depend on sodium (Na+) and potassium (K+) levels. At high potassium concentrations and low sodium concentrations, the synthesis and secretion of aldosterone is enhanced. The most important influence on aldosterone levels is the renin-angiotensin system (see RAAS). Other factors also influence aldosterone levels. Many factors are involved in the development of hyperkalemia, which develops as a result of a decrease in potassium excretion or an increase in the release of potassium from cells.
Hyperkalemia may be false (pseudohyperkalemia), this should be excluded first (except in cases where emergency assistance is required).
Pseudohyperkalemia
Pseudohyperkalemia is a condition when the calcium level determined in the laboratory does not reflect the level of potassium in the body.
This is because intracellular potassium levels are very high and in certain situations it is released from the cells after blood is drawn. In such cases, to confirm true hyperkalemia, blood sampling should be repeated and plasma and serum potassium levels should be measured simultaneously. The concentration in the serum is 0.2–0.4 mmol/l higher than the concentration in plasma, which is associated with the formation of a clot and the release of potassium from cells into the serum. Table 1: Causes of pseudohyperkalemia
- Untimely analysis
- Taking blood from a vein into which potassium was injected
- Using too much pressure when applying a tourniquet or using a fist to fill the veins
- Hemolysis due to blood flow through a thin needle or traumatic venipuncture
- Long-term blood storage
- High leukocytosis or thrombocytosis (significantly increased white blood cell or platelet count)
- Unusual genetic disorders (familial hyperkalemia)
Hyperkalemia with increased potassium intake
Excessive dietary potassium intake may contribute to hyperkalemia if urinary potassium excretion is simultaneously reduced. With normal kidney function, all potassium should be excreted.
Table 2: Foods High in Potassium
- Salt substitutes
- Figs
- Syrup
- Bran, cereals, wheat germ
- Vegetables (spinach, tomatoes, carrots, potatoes, broccoli, lima beans, cauliflower) and mushrooms
- Dried fruits, nuts, seeds
- Fruits (bananas, kiwi, oranges, mango, melon)
Hyperkalemia may be associated with blood transfusion - intravenous administration of blood cells, from which potassium is released into the extracellular space, with too rapid administration of calcium preparations to treat hypokalemia, with high potassium content during parenteral nutrition.
Hyperkalemia associated with the release of potassium from cells
Some exogenous and endogenous factors can disrupt the exchange of potassium between intercellular and intracellular fluids and increase the concentration of potassium in the serum. However, this mechanism rarely causes severe hyperkalemia unless the factor is, for example, tissue damage, necrosis (local tissue death as a result of injury), rhabdomyolysis, tumor breakdown, severe burns.
Table 3: reasons for potassium redistribution Redistribution of potassium between extracellular and intracellular fluids
- Muscle necrosis, myolysis (rhabdomyolysis - damage to skeletal muscles), tumor breakdown, severe burns
- Insulin deficiency (normally, this hormone accelerates the movement of potassium into cells)
- Metabolic acidosis
- Hyperosmolarity (hyperglycemia - increased blood glucose levels, administration of mannitol)
- Medicines (eg, succinylcholine (aka dithiline, listenone), beta blockers, digoxin)
- Hyperkalemic periodic paralysis (attacks most often develop 30-40 minutes after exercise)
Decreased potassium excretion
- Kidney damage (glomerular filtration <20 ml/min)
- Decreased mineralcorticoid activity
- Hyporeninemic form of hypoaldosteronism (chronic kidney disease, diabetic nephropathy, NSAIDs)
- Adrenal insufficiency (Addison's disease, congenital enzyme defect)
- Aldosterone blockers (see table 4)
- Immunity to aldosterone (sickle cell anemia, systemic lupus erythematosus, amyloidosis, obstructive nephropathy)
Decreased rate of urine filtration and sodium delivery to the distal nephron
- Hypovolemia
- Some genetic disorders, such as Gordon's syndrome
Hyperkalemia caused by impaired potassium excretion
Most potassium is excreted by the kidneys, so kidney damage is the main cause of hyperkalemia. Kidney damage accounts for up to 75% of cases of this condition.
In patients with chronic kidney disease, the ability to excrete potassium is maintained until renal failure develops (when filtration is reduced to less than 15-20 ml/ml) or when the patient consumes large amounts of potassium or takes drugs that increase potassium levels .
Damage to the juxtaglomerular apparatus leads to a deficiency in renin production (see RAAS), which causes hyponatremia and hypoaldosteronism, which can also cause hyperkalemia even in the absence of severe kidney damage. Hyporeninemic hypoaldosteronism is also known as type 4 tubular acidosis type 4, as it is often associated with mild to moderate metabolic acidosis, with a normal anion gap (the difference between cations and anions; Anion gap = (Na + K) - (Cl + [HCO3- ]) (units – mol/l)). Most often, this condition develops with diabetic nephropathy.
Hypoaldosteronism may also be a consequence of primary diseases of the adrenal glands (Addison's disease, congenital disorders of steroid synthesis, 21-hydroxylase deficiency) or a decrease in mineralcorticoid activity. The latter problem is often associated with sickle cell anemia, systemic lupus erythematosus, amyloidosis, obstructive nephropathy, and the use of potassium-sparing diuretics. In rare cases, a mutation of the mineralcorticoid receptor gene is detected.
In general, disturbances in mineralcorticoid metabolism do not cause hyperkalemia if normal amounts of sodium enter the distal nephron. Thus, patients with Addison's disease do not always develop hyperkalemia if they have adequate salt intake. Impaired urinary excretion or sodium delivery to the distal nephron plays an important role in the development of hyperkalemia. These disorders can be caused by internal factors or (more often) by certain medications (see tables 3, 4).
Drugs that cause hyperkalemia
Drugs can disrupt potassium homeostasis through several mechanisms: activation of transmembrane potassium transport, reduction in renal excretion (changes in the action of aldosterone, sodium delivery to the distal nephron, changes in the function of the collecting ducts). The risk increases when such drugs are taken by patients with kidney failure. Elderly patients and patients with diabetes are especially susceptible to developing hyperkalemia. In these groups, such drugs should be used with caution, starting with small doses and monitoring potassium levels whenever the dose is changed. There are no recommendations regarding the number of studies; the frequency of determining potassium levels depends on the level of renal failure, the presence of diabetes, medications taken, and concomitant diseases.
In particular, it is necessary to approach the management of patients with impaired electrical conductivity of the heart muscle very carefully, since even a slight increase in potassium levels can lead to arrhythmia.
The full list of medications is in the table.
Drugs that interfere with potassium transmembrane transport
- Beta blockers
- Digoxin
- Hyperosmolar solutions (mannitol, glucose)
- Suxamethonium (Listenon)
- Intravenous administration of positively charged amino acids
Substances containing potassium
- Potassium supplements
- Salt substitutes
- Herbal preparations (alfalfa, dandelion, horsetail, spurge, nettle)
- Red blood cells (when they break down, potassium is released)
Medicines that reduce aldosterone secretion
- ACE inhibitors
- Angiotensin II receptor blockers
- NSAIDs (NSAIDs)
- Heparin preparations
- Antifungal drugs (ketoconazole, fluconazole, intraconazole)
- Cyclosporins
- Prograph
Drugs that block the binding of aldosterone and mineralcorticoid receptors
- Spironolactone
- Inspra (Eplerenonum)
- Drospirenone
Drugs that inhibit potassium channel activity in epithelial cells
- Potassium-sparing diuretics (amiloride, triamterene)
- Trimethoprim (an antimicrobial drug)
- Pentamidine (antimicrobial)
Let's look at some of them:
Angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are widely used to protect the kidneys and reduce mortality from heart disease, especially in diabetics. They are also included in standard treatment regimens for patients with chronic heart disease.
These drugs predispose to the development of hyperkalemia because they impair aldosterone secretion and reduce renal reperfusion (and glomerular filtration rate). Both drugs reduce urinary potassium excretion.
They do not cause hyperkalemia in patients with normal renal function; The degree of suppression of aldosterone secretion is not sufficient to significantly impair potassium excretion unless there is previous hypoaldosteronism (due to some disease or other medications). Unfortunately, people taking these drugs are at high risk of developing hyperkalemia. About 10% of outpatients develop hyperkalemia within a year of starting treatment with AFP inhibitors or angiotensin II receptor blockers.
Moreover, these drugs contribute to the development of hyperkalemia in 10-38% of patients admitted to hospitals. The risk is especially increased if the patient is taking high doses of drugs or in combination with other drugs that cause hyperkalemia.
Aldosterone (mineralocorticoid) receptor antagonists are also frequently used in the treatment of patients with congestive cardiac failure since the Randomized Aldactone Evaluation Study showed that the addition of spironolactone to treatment reduced morbidity and mortality. This study indicated that severe hyperkalemia developed in only 2% of patients when the creatinine concentration was 106 mmol/L and the spironolactone dose did not exceed 25 mg per day. In contrast, population based time-series analyzes demonstrate a significant increase in hospitalization and mortality from hyperkalemia. This is likely because the study included patients with severe renal impairment who were taking high doses of spironolactone. These patients were more likely than others in the study to take potassium supplements or other drugs that interfere with potassium excretion. The risk increases when spironolactone is combined with AFP inhibitors and ARBs, especially in elderly patients with impaired renal function.
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit renin secretion (leading to hypoaldosteronism and decreased renal potassium excretion) and may impair renal function.
These drugs may be given judiciously in patients with diabetes or renal failure, especially if patients are taking other drugs (ACE inhibitors and ARBs). How is hyperkalemia diagnosed?
Hyperkalemia often occurs without symptoms and is detected during laboratory testing. When symptoms exist, they are not specific and are predominantly associated with disturbances in muscle function (paresthesia, muscle weakness, fatigue) or cardiac function (palpitations).
ECG: high “peaked” T wave, flattening or absence of P wave, widening of the QRS complex, sine waves.
However, ECG is not a sensitive method for diagnosing hyperkalemia. In a study by Acer et al, nearly half of patients with potassium levels above 6.5 mmol/L had no ECG changes. In addition, some patients experience a gradual change in cardiac function, while others experience a rapid progression from benign changes to fatal ventricular arrhythmias.
Evaluation of patients with hyperkalemia should include:
- Careful review of medical history to identify potential risk factors such as renal impairment, diabetes, adrenal insufficiency, use of medications that may cause hyperkalemia.
- Laboratory tests should be aimed at confirming the medical history and physical examination, and should include urea, creatinine, electrolytes, osmolarity (an acute increase in osmolarity can cause potassium to leak out of cells), and urine potassium concentration.
- In some patients, additional specific tests are performed, such as fractional sodium excretion or a transtubular potassium gradient may be used to distinguish between renal and nonrenal causes of hyperkalemia.
What to do with severe hyperkalemia?
Guidelines for the management of hyperkalemia are based on expert opinion due to a lack of clinical research. Treatment should be aimed at restoring electrolyte metabolism, preventing serious complications and treating the underlying disease. Figure In the management of mild to moderate hyperkalemia, loop diuretics are used to increase potassium excretion. Potassium intake from food should be limited. The dose of medications that increase potassium levels in the blood should be reduced or discontinued as much as possible. If the patient has impaired renal function, diuretics may not be effective. Then other measures are required, in particular dialysis.
Severe hyperkalemia is a life-threatening condition as it can cause serious disturbances of the heart and neuromuscular system, including cardiac arrest and paralysis of the respiratory muscles. Therefore, urgent and aggressive therapy is required. Many authors provide the following criteria for severe hyperkalemia: more than 6.0 mmol/l + changes on the ECG, or more than 6.5 mmol/l regardless of the presence of changes on the ECG.
If there are signs of hyperkalemia on the ECG or in the event of cardiac arrest in patients on dialysis, for example, therapy is started without laboratory data. Other factors that require proactive treatment: rapid rise in potassium levels, signs of acidosis, rapid deterioration of kidney function.
Most guidelines and experts advise that severe hyperkalemia should be treated in a hospital setting, which allows for continuous cardiac monitoring, since even patients without symptoms or ECG changes can develop life-threatening arrhythmias.
Although emergency dialysis removes potassium from the body, it is urgent to begin treatment of the underlying disease, as the 2005 Cochrane systematic review of emergency interventions for hyperkalaemia recommends doing three things :
- The first step is to stabilize myocardial activity and reduce the likelihood of arrhythmia. Intravenous calcium is used as a direct antagonist of potassium in influencing the membrane, stabilizing cardiac conduction. Calcium gluconate in a volume of 10 ml of a 10% solution is administered over 3-5 minutes under the control of the heart on a cardiac monitor. Calcium infusion has no effect on serum potassium levels, but does affect cardiac function: changes on the ECG are visible within 1-3 minutes after calcium administration, the effect lasts for 30-60 minutes. The infusion may be repeated if there is no effect within 5-10 minutes. Calcium should be used very carefully in patients taking digoxin, as calcium increases the toxic effects of digoxin.
- The second step is the transfer of potassium from extracellular fluid to intracellular fluid. This reduces serum potassium levels. This is achieved by administering insulin or beta-2 agonists, which stimulate the sodium-potassium pump. Insulin is administered intravenously as a bolus with sufficient glucose (to prevent hypoglycemia). The effect of insulin administration occurs after 20 minutes, reaches a maximum after 30-60 minutes, and lasts up to 6 hours. The most commonly used selective beta-2 agonist is salbutamol. Salbutamol is used using a nebulizer. The effect occurs after 20 minutes, the maximum effect is at 90-120 minutes. Salbutamol can be used alone or in combination with insulin. Sodium bicarbonate may also be prescribed to patients with acidosis, although the benefit of this drug for hyperkalemia remains controversial.
- Thirdly , measures are taken to remove potassium from the body. Potent loop diuretics (eg, 40 to 80 mg furosemide IV) increase renal potassium excretion by increasing urine production and sodium delivery to the distal nephron. But diuretics only work if kidney function is preserved, and many patients with hyperkalemia have acute or chronic kidney disease. Cation exchange resins, which remove potassium from the extracellular fluid in exchange for sodium through the intestine, are also widely used, although their effectiveness is controversial. They work faster as an enema than when administered orally. It may take 6 hours to achieve the effect. Dialysis is the definitive treatment for patients with severe hyperkalemia and progressive kidney disease.
Long-term management of hyperkalemia
After emergency treatment, measures should be taken to prevent recurrence of hyperkalemia.
The first step is to analyze the medications the patient is taking. If possible, reduce or eliminate medications that increase potassium levels. Because AFP inhibitors and angiotensin II receptor blockers slow the progression of chronic kidney disease, using other measures to control hyperkalemia or reducing the drug dose is preferable to stopping these drugs. It is wise to limit your potassium intake to 40-60 mmol per day. The use of diuretics may also be effective. Thiazide diuretics can be used in patients with preserved renal function, but they are usually ineffective if the glomerular filtration rate is less than 40 ml/min, when loop diuretics are more preferable. Fludrocortisone may be used in patients with hyporeninemic hypoaldosteronism. However, this drug should be used with caution, especially in patients with type 2 diabetes, which often occurs with hypertension, as the drug leads to fluid retention and increased blood pressure. Tips for non-experts
- In patients without a predisposition to hyperkalemia, repeat the potassium test to rule out pseudohyperkalemia.
- Don't forget about possible hidden causes of hyperkalemia, including NSAIDs
- ACE inhibitors and angiotensin II receptor blockers should be started at low doses, and potassium levels should be monitored one week after starting treatment and after dose increases.
- All patients with hyperkalemia should undergo a 12-lead ECG.
- ECG changes and arrhythmias associated with hyperkalemia require urgent medical attention.
Questions for current and future researchers
- How important is the contribution of various risk factors to the development of hyperkalemia and how to evaluate them?
- With the increasing use of cardio- and renoprotectors that promote potassium retention, which method is most preferable for monitoring potassium levels?
Improved understanding of the molecular mechanisms underlying the maintenance of extracellular fluid potassium levels, developing new therapeutic strategies for the treatment of life-threatening hyperkalemia.
| Spironolactone – a potassium-sparing diuretic, a competitive antagonist of aldosterone in its effect on the distal nephron. Increases the excretion of Na+, Cl- and water and reduces the excretion of K+ and urea. Trade names: Veroshpiron, Spironolactone. Contraindications: Addison's disease, hyperkalemia, hypercalcemia, hyponatremia, chronic renal failure, anuria, liver failure, diabetes mellitus with confirmed or suspected chronic renal failure, diabetic nephropathy, first trimester of pregnancy, metabolic acidosis, menstrual irregularities or breast enlargement, hypersensitivity to spironolactone. More details on the website vidal.ru: | Exogenous – literally translated, formed from the outside. In medicine it is used in relation to the body and means that a certain factor is formed and comes from the outside. For example, exogenous factors are food. Endogenous is the opposite term, meaning that a certain factor is formed inside the body. |
RAAS – renin-angiotensin-aldosterone system. When the pressure in the kidneys decreases (bleeding, fluid loss, decreased sodium chloride concentration), an enzyme is produced in the juxtaglomerular cells of the kidneys renin. The substrate of this enzyme (the substance on which the enzyme acts) is angiotensinogen. Under the influence of renin, angiotensinogen is converted into angiotensin I. Angiotensin II is formed from angiotensin II under the action of angiotensin-converting enzyme (ACE) and has the following actions:
Hereditary predisposition to severe vascular spasm, including those associated with ACE, is determined using the block of analyzes “Polymorphism of vascular tone genes.” | ECG - electrocardiography - a study based on recording the electric fields generated during the work of the heart using a special device - an electrocardiograph. The ECG is recorded using special electrodes placed on certain areas of the body. ECG is a common method for assessing heart function. ECG analysis, ABC ECG analysis, practice: https://www.practica.ru/BK1/5.htm |
| Acidosis (from Latin acidus - sour) is a change in the acid-base state associated with increased acidity (increased hydrogen ions). Acidosis is said to occur when arterial blood pH falls below 7.35. Contrasted with alkalosis, when the pH is greater than 7.45. Metabolic acidosis is associated with metabolic disorders (excessive formation of acids, insufficient excretion, loss of bases). Respiratory (respiratory) acidosis develops when pulmonary ventilation is impaired (respiratory failure or circulatory failure in the lungs). | Geodialysis (dialysis) is a method of extrarenal blood purification in case of renal failure. Hemodialysis is carried out using an artificial kidney machine. |
| Homeostasis - in physiology - the ability of a self-regulating system to maintain a constant internal state through coordinated reactions aimed at maintaining dynamic constancy. The term was proposed by the American physiologist W. Cannon in 1929. Examples of homeostasis are maintaining blood pH, body temperature, pressure, and so on. | Parenteral nutrition is the administration of nutrients (carbohydrates, amino acids, lipids, salts, vitamins) intravenously when natural food intake is not possible. |
| Gordon's syndrome is a rare disease associated with increased chloride reabsorption in the distal nephron. Characterized by the presence of hyperkalemia, metabolic acidosis, hyporeninemia, hypoaldosteronemia, decreased sensitivity of the kidneys to mineralcorticoids. | |
| Mineralcorticoids are a group of corticosteroid hormones of the adrenal cortex that affect water-salt metabolism (aldosterone, deoxycorticosterone). | |
| The juxtaglomerular apparatus of the kidneys (JRA) is a collection of renal tissue cells that form and secrete biologically active substances, including renin. |
Tags:
hyperkalemia, potassium, electrolytes
Back to section
ELECTROPHYSIOLOGICAL EFFECTS OF HYPOKALEMIA
Hypokalemia leads to an increase (more electronegative value) of the membrane PP and, at least during electrical diastole, reduces membrane excitability due to an increase in the difference between the PP and the threshold potential. It is assumed that extracellular potassium is necessary for the opening of delayed rectifying current channels [31]. Low extracellular potassium levels reduce the delayed (outward) potassium current, leading to increased AP duration and slower repolarization. The most important here is the disruption of the AP configuration, especially the slowing down of the “slope” of the 3rd phase of repolarization. An AP with a long “tail” is formed, which leads to an increase in the relative refractory period (RPP) and a decrease in the difference between the AP and the threshold potential in the final phase of the AP. Therefore, the myocardium exhibits increased excitability and an associated tendency to ectopic activity during a significant portion of the AP. Conduction slows as depolarization begins in incompletely repolarized fibers. Moreover, hypokalemia lengthens the plateau phase in Purkinje fibers, but shortens it in ventricular fibers [10]. The repolarization phase (“tail”) of AP in the conduction system is prolonged more than in the ventricles, which increases the spread of repolarization. Hypokalemia accelerates diastolic depolarization in Purkinje fibers, thereby increasing automaticity. In total, the electrophysiological effects of hypokalemia are manifested in a decrease in conduction velocity, shortening of the effective refractory period (ERP), prolongation of the ORP, increased automaticity and early afterdepolarization.
ECG manifestations of hypokalemia:
- due to changes in repolarization:
– decrease in amplitude and expansion of the T wave; – noticeable wave U; – reduction of the ST segment; – fusion of T and U waves (with severe hypokalemia);
- due to conduction disorders:
– increase in the duration of the QRS complex; – atrioventricular block; – increase in amplitude and expansion of the P wave; – slight increase in the P–R interval; - cardiac arrest.
When the U wave exceeds the T wave in amplitude, the plasma potassium level is <3 mmol/L (see figure).
ARRHYTHMOGENIC POTENTIAL AND CLINICAL CONSEQUENCES OF HYPOKALEMIA
The increase in excitability caused by hypokalemia is clinically manifested by the development of supraventricular and ventricular extrasystole. Decreased potassium and magnesium levels have been shown to correlate with an increased incidence of premature ventricular complexes [30]. Hypokalemia contributes to the development of the reentry phenomenon through slowing conduction against the background of prolongation of the ORP, which leads to an increase in the spread of refractoriness. The suppressive effect of hypokalemia on the operation of the Na+-K+ pump leads to excessive accumulation of Ca++ in the cell, which contributes to the development of delayed afterdepolarization through a transient inward current. In the experiment, hypokalemia increased the tendency to ventricular fibrillation in both normal and ischemic myocardium [12]. A connection has been established between hypokalemia and ventricular fibrillation in patients with acute myocardial infarction [7, 18]. Hypokalemia increases the binding of cardiac glycosides to Na+-K+-adenosine triphosphatase (ATPase), reduces the rate of elimination of digoxin and potentiates the toxic effects of digitalis preparations. When using potassium preparations, it is necessary to remember one important phenomenon - the Zwaardemaker-Libbrecht effect, which develops as a result of a rapid increase in the level of extracellular potassium from low to high and manifests itself as a short-term stop of pacemaker cells, a decrease in the duration of AP and hyperpolarization [13] . This phenomenon highlights the fact that the rate of intravenous potassium administration is more important in terms of arrhythmogenic effects than the absolute amount of potassium administered and the final level of extracellular potassium.
MAGNESIUM
Ionized magnesium is in 2nd place after potassium in terms of total content in the cell. The magnesium content in blood plasma is 0.8–1.5 mmol/l; in muscle tissue its content is 10 times higher than in plasma. Thanks to this depot of magnesium in the muscles, its level in the blood can remain stable for a long time even with significant losses. The significance of disturbances in magnesium metabolism is still debated due to the difficulties of measuring it and the common association of these disturbances with other electrolyte disorders [14, 27]. Magnesium is involved in the functioning of muscles and the nervous system, in the regulation of heart rate, cholesterol, lipid, phosphorus and calcium metabolism. It serves as an important cofactor for numerous enzymatic reactions involved in nucleotide and carbohydrate metabolism, protein synthesis and other processes also necessary for normal cardiovascular physiology. Magnesium prevents an increase in blood pressure, enhances inhibition processes in the central nervous system, causing sedative, tranquilizing effects and preventing the manifestations of convulsive activity. Being a natural antagonist of Ca++, magnesium reduces blood clotting and vascular tone [2, 4]. The daily requirement of an adult for magnesium is from 300 to 500 mg. Magnesium deficiency occurs frequently, but its electrophysiological consequences for the myocardium elude scientists even with the most careful study. It is known that the use of magnesium preparations (magnesium) in pharmacological doses is useful for the treatment of torsades de pointes. Toxic effects of magnesium are rare (with the exception of patients with impaired renal function). With magnesium deficiency, nervousness, irritability, and sleep disturbances develop against the background of muscle weakness, increased fatigue and paresthesia. With chronic magnesium deficiency, skeletal deformations are possible: scoliosis, funnel chest, flat feet.
Causes of seizures
There are several factors that influence the occurrence of seizures.
Occupational activities - People whose jobs involve standing in a single position all the time (salespeople, computer operators, etc.) or who are exposed to repetitive physical stress experience cramps at night or in the evening. People who smoke experience muscle cramps 5 times more often; the leading causes of cramps are smoking or extremely frequent drinking of coffee.
Often night cramps appear after a day spent in constant movement or even an ordinary brisk walk - this becomes the cause of calf cramps. The same result causes unusual significant physical stress. It should be taken into account in advance that it is undesirable to strain one muscle group; physical work should alternate with periodic rest.
Night cramps are much more common because the posture of the sleeping person contributes to this: relaxed and downward-facing feet, bent legs and the body turned on its side. This position shortens the calf muscle and helps cause spasms. Night leg cramps occur in athletes after significant daytime stress, children (especially with fever) and the elderly due to circulatory disorders in the extremities.
Severe cramps are not uncommon when swimming in cool water on a hot day. Temperature changes can also cause spasms in the pool: heated muscles contract sharply with sudden changes in the environment.
Cramps also appear as a result of severe dehydration, especially in those who engage in sports training or heavy physical labor in the hot season.
Stressful conditions can cause convulsions, and the effect does not occur immediately, but after a while, when calcium is redistributed in the body due to an excess of the hormone cortisol.
Spasms of the fibers of the foot muscles can appear as a result of wearing tight shoes or high loads with flat feet.
Another reason that leads to cramps in the arms and legs is the uncontrolled use of statins, dauretics, antibiotics and other drugs that redistribute or remove microelements from the body. Drug treatment of seizures is carried out with mandatory monitoring of the biochemical composition of the blood.
ELECTROPHYSIOLOGICAL EFFECTS AND ECG MANIFESTATIONS OF HYPOMAGNESIEMIA
At very low extracellular calcium concentrations, magnesium affects the transmembrane current or currents that modulate the duration of the plateau phase of AP in the ventricles. It has been established that at normal calcium concentrations, magnesium deficiency has a negligible effect on the PP of the papillary muscle of the heart of dogs [11]. However, when the calcium concentration decreases to 1/10 of normal, complete removal of magnesium from the perfusion solution prolongs the plateau phase of AP, which was already prolonged due to low calcium concentration, from normal values (100–150 ms) to 1000 ms or more. Magnesium blocks calcium channels, shifts the inactivation curve of fast sodium channels in the direction of hyperpolarization, modulates the effects of hyperkalemia, and modulates potassium currents. In a study in healthy patients, the following ECG effects of intravenous magnesium administration were noted: significant prolongation of the P–R interval; lengthening of the conduction interval from the atria to the His bundle; increase in sinoatrial conduction time; Elongation of the ERP in the atrioventricular node [16]. Hypermagnesemia reduces atrioventricular and intraventricular conduction. Neither hypermagnesemia nor hypomagnesemia causes any specific ECG changes. Intravenous administration of magnesium sulfate to patients with a prolonged QT interval and torsade de pointes (TdP) may reverse ventricular tachycardia if baseline magnesium levels are normal or low. O. Takanaka et al. [28] studied the effects of magnesia and lidocaine on AP duration and barium-induced early afterdepolarization in canine Purkinje fibers. Their data confirm that hypomagnesemia can have an arrhythmogenic effect when combined with hypokalemia and bradycardia; under these conditions, the administration of magnesium can suppress trigger activity, mainly directly preventing the development of trigger APs. There were no specific electrophysiological effects or arrhythmias associated with isolated magnesium deficiency. However, magnesium may influence the development of cardiac arrhythmias through direct effects or by modulating the effects of potassium or acting as a calcium channel blocker. It is known that magnesium deficiency has a negative effect on the normal functioning of membrane ATPase, slowing down the transfer of sodium from the cell and potassium into the cell. This disrupts the transmembrane equilibrium of potassium and can lead to changes in membrane PP, changes in potassium transmembrane conductance, and disturbances in the repolarization phase [9]. There is evidence that dietary magnesium intake may have a moderate inverse correlation with the risk of developing coronary heart disease, particularly in men [5].
Types of seizures
All categories of people can experience seizures, regardless of age and lifestyle; they appear more often in the elderly and young children. Attacks are manifested by unequal painful muscle contractions and have different durations, mechanisms of development and frequency of occurrence. They are classified according to several criteria.
By locality
Depending on whether one muscle or a group of them is seized by a spasm, spasms are divided into:
- unilateral – when pain occurs on one side;
- focal (local) - appear in one muscle group;
- generalized - affect the whole body, causing involuntary urination, loss of consciousness and even respiratory arrest.
By duration of exposure
Classified into:
- myoclonic - short-term contractions or twitches, mainly in the upper part of the body, stop quickly and do not cause pain;
- clonic - characterized by a longer duration, muscles contract rhythmically, can be of a local or general type, sometimes contribute to stuttering;
- tonic – long-term, can occur in any part of the body, affect the respiratory tract, the part of the body seized by a convulsion takes one forced position, can lead to loss of consciousness;
- tonic-clonic - in which tonic spasms turn into clonic; if the second type predominates, they are called clono-tonic.
USE OF POTASSIUM AND MAGNESIUM PREPARATIONS IN CLINICAL PRACTICE
Briefly summarizing the above, it must be said that potassium and magnesium play an important role in the regulation of the functions of the cardiovascular system and central nervous system, ensure the normal course of numerous metabolic biochemical processes in almost all tissues and organs, regulate neuromuscular transmission, have a positive effect on phosphorus-calcium metabolism and support electrolyte balance in the body. Therefore, preparations of these macroelements can and should be used for a very wide range of indications - both in the treatment of various pathological conditions and for prophylactic purposes, primarily to prevent hypokalemia and hypomagnesemia in patients taking diuretics (thiazide and, especially, loop diuretics ) and (or) cardiac glycosides (digitalis preparations). In practical medicine, preparations containing one of these elements are used - potassium (potassium chloride, potassium orotate) or magnesium (Magne B6, Magnerot, magnesium sulfate), as well as complex preparations containing both cations (potassium aspartate + magnesium aspartate: Panangin, asparkam , potassium and magnesium aspartate BerlinChemie). In this series, one of the most effective and balanced in composition preparations of potassium and magnesium aspartate, solid experience of successful use of which has been accumulated by domestic and world medical practice, is Panangin. The combined administration of potassium and magnesium salts provides the advantage of a potentiating effect of their interaction. This combination is all the more justified since the metabolism of potassium and magnesium is closely related and clinically significant hypomagnesemia usually develops against the background of hypokalemia or complicates existing hypokalemia. Aspartic acid, which is part of Panangin, being a natural amino acid, easily penetrates the cell and facilitates the entry of potassium and magnesium into it. It is also useful because it is part of many proteins, plays an important role in the metabolism of nitrogenous substances, participates in transamination reactions and in the formation of pyrimidine bases of nucleic acids. Despite the real achievements of medical science in recognizing the etiopathogenesis of CVD, their prevalence is steadily growing, they remain the leading cause of disability and mortality in the population both in Russia and throughout the world. Therefore, the most relevant indications for the use of Panangin remain diseases such as chronic heart failure, ischemic heart disease, including myocardial infarction, cardiac arrhythmias, especially associated with taking digitalis drugs, and their combinations. Panangin is the drug of choice in the prevention and replenishment of potassium and magnesium deficiency and in other clinical situations. In the future, the indications for the use of Panangin will expand. Information is accumulating on the benefits of using potassium and magnesium for the treatment and prevention of cerebrovascular accidents and hypertension. Thus, replacing regular table salt with salt fortified with potassium and magnesium in individuals with normal or moderately elevated blood pressure led to a significant decrease in sodium intake and a decrease in systolic blood pressure [22]. A study by A. Ascherio et al., which included more than 43 thousand men aged 40 to 75 years, found a significantly lower relative risk of stroke (0.62) in people with high potassium intake (average - 4. 3 g/day) than in individuals with low consumption (on average 2.4 g/day) [6]. There was also an inverse correlation between magnesium intake (but not calcium intake) and the relative risk of stroke. All of these correlations were more pronounced in men with arterial hypertension than in normotensive individuals. The authors concluded that potassium supplementation may be more widely used in patients with hypertension. However, this information cannot justify the indiscriminate prescription of potassium supplements to everyone, since their uncontrolled use can be dangerous. Magnesium intake from drinking water has been reported to statistically significantly reduce the risk of death from cerebrovascular disease [32]. One prospective study showed that an increase in potassium intake by 10 mmol/day was accompanied by a significant reduction in the risk of death from stroke by 40% [15]. This effect was independent of other risk factors or variables, including magnesium intake. A recent meta-analysis of prospective studies found a statistically significant inverse correlation between potassium intake and the risk of stroke, especially ischemic stroke [17]. For every 1,000 mg increase in potassium intake, the risk of stroke decreased by 11%. Almost simultaneously with this work, another meta-analysis was published in the Journal of the American College of Cardiology, which reported a 21% reduction in the risk of stroke when taking a potassium supplement in a daily dose of 1.42 g and a significant reduction in the risk of coronary events and cardiovascular complications in general [8]. Potassium and magnesium preparations are also used in surgical anesthesiology. R. Soave et al. report that the use of magnesium in anesthesiological practice not only provides an antinociceptive effect due to the blockade of NMDA receptors and associated channels [33], but is also necessary to correct magnesium deficiency, which in its pure form was observed in 7–11% of hospitalized patients, and in combination with other electrolyte disturbances, especially hypokalemia and hypophosphatemia, in more than 40% of patients [25]. Replenishment of magnesium deficiency in this case, according to the observations of the authors, is necessary to prevent an increase in morbidity and mortality in the postoperative period. The administration of potassium and magnesium aspartate is especially important during operations on the heart and large vessels, since a significant decrease in the level of potassium and magnesium is also observed during extracorporeal circulation [3]. If potassium and magnesium aspartate were not pre-administered, hypomagnesemia developed in 50% of patients. Thus, potassium and magnesium preparations, including Panangin, are increasingly used in everyday clinical practice.
Literature 1. Vislobokov A.I., Ignatov Yu.D., Melnikov K.N. Pharmacological regulation of ion channels of neuronal membranes / St. Petersburg: Publishing house of St. Petersburg State Medical University. – 2006; 288 p. 2. Lesiovskaya E.E., Bakhtina S.M., Boyko I.N. Vitamins, macro- and microelements / St. Petersburg: publishing house of St. Petersburg KhFA. – 2004; 140 pp. 3. Trekova N.A., Andrianova M.Yu., Tolstova I.A., et al. The use of a solution of potassium and magnesium aspartate to maintain the balance of potassium and magnesium during cardiac surgery under artificial circulation // Anesthesia. and resuscitator. – 2008; 5:17–21. 4. Shestakova S.A., Dolgodvorov A.S., Kubynin A.N. Disturbances of water-electrolyte metabolism and their pharmacological correction / St. Petersburg: Publishing house of St. Petersburg State Medical University. – 2005; 91 p. 5. Al-Delaimy W., Rimm E., Willett W. et al. Magnesium intake and risk of coronary heart disease among men // J. Am. Coll. Nutr. – 2004; 23 (1): 63–9. 6. Ascherio A., Rimm E., Hernan M. et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men // Circulation. – 1998; 98:1198–204. 7. Cooper W., Kuan P., Reuben S., Vanderburg M. Cardiac arrhythmias following acute myocardial infarction: Assosiation with serum potassium level and prior diuretic therapy // Eur. Heart J. - 1984; 5:464–9. 8. D'Elia L., Barba G., Cappuccio F., Strazzullo P. Potassium intake stroke and cardiovascular disease. A meta-analysis of prospective studies // J. Am. Coll. Cardiol. – 2011; 57:1210–19. 9. Dykcner T., Wester P. Relation between potassium, magnesium and cardiac arrhythmias // Acta Med. Scand. Suppl. – 1981; 647:163–9. 10. Gettes L., Surawicz B. Effects of low and high concentrations of potassium on the simultaneously recorded Purkinje and ventricular action potentials of the perfused pig moderator band // Circ. Res. – 1968; 23: 717–29. 11. Hoffman B., Suckling E. Effect of several cation on transmembrane potentials of cardiac muscle // Am. J. Physiol. – 1956; 186:317–24. 12. Hohnloser S., Verrier R., Lown B., Reader E. Effect of hypokalemia on susceptibility to ventricular fibrillation in the normal and ischemic canine heart // Am. Heart J. - 1986; 112:32–5. 13. Ito S., Surawicz B. Transient, “paradoxical” effects of increasing extracellular K+ -concentration on transmembrane potential in canine cardiac Purkinje fibers // Circ. Res. – 1977; 41: 799–807. 14. Keller P., Aronson R. The role of magnesium in cardiac arrhythmias // Prog. Cardiovasc. Dis. – 1990; 32:433–48. 15. Khaw K., Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study // N. Engl. J. Med. – 1987; 316:235–40. 16. Kulick D., Hong R., Ryzen E. Electrophysiologic effects of intravenous magnesium in patients with normal conduction systems and no clinical evidence of significant cardiac disease // Am. Heart J. - 1988; 115:367–73. 17. Larsson S., Orsini N., Wolk A. Dietary potassium intake and risk of stroke. A dose-response meta-analysis of prospective studies // Stroke. – 2011; 42 (10): 2746–50. 18. Nordrehaug J., Johanessen K., Von der Lippe G. Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction // Circulation. – 1985; 71:645–49. 19. Paice B., Paterson K., Onyanga-Omara F. et al. Record linkage study of hypokaliemia in hospitalized patients // Postgrad. Med. J. – 1986; 62:187–91. 20. Pelzer D., Trautwein W. Currents through ionic channels in multicellular cardiac tissue and single heart cells // Experentia. – 1987; 43: 1153–62. 21. Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea pig heart // J. Physiol. – 1984; 347:641–57. 22. Sarkkinen E., Kastarinen M., Niskanen T. et al. Feasibility and antihypertensive effect of replacing regular salt with mineral salt – rich in magnesium and potassium – in subjects with mildly elevated blood pressure // Nutr. J.– 2011; 10: 88. 23. Schulman M., Narins R. Hypokalemia and cardiovascular disease // Am. J. Cardiol. – 1990; 65:4–9. 24. Sheu S, Korth M, Lathrop DA et al. Intra- and extra-cellular K+ and Na+ activities and resting membrane potential in sheep cardiac Purkinje strands // Circ. Res. – 1980; 47: 692–700. 25. Soave PM, Conti G., Costa R., Arcangeli A. Magnesium and anesthesia // Curr. Drug Targets. – 2009; 10 (8): 734–43. 26. Surawicz B. Relation between electrocardiogram and electrolites // Am. Heart J. – 1967; 73:814–43. 27. Surawicz B., Lepeschkin E., Herrlich H. Low and high magnesium concentrations at various calcium levels: Effect on the monophasic action potential, electrocardiogram and contractility of isolated rabbit hearts // Circ. Res. – 1961; 9: 811–8. 28. Takanaka C., Ogunyankin K., Sarma J., Singh B. Antiarrhythmic and arrhythmogenic actions of varying levels of extracellular magnesium: Possible cellular basis for the differences in the efficacy of magnesium and lidocaine in torsades de pointes // J. Cardiovasc . Pharmacol. Ther. – 1997; 2: 125–34. 29. Thompson R. Gobb L., Hypokalemia after resuscitation out-of-hospital ventricular fibrillation // JAMA. – 1982; 248:2860–3. 30. Tsuji H., Venditti F., Evans J., et al. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study) // Am. J. Cardiol. (US). – 1994; 74:232–5. 31. Yang T., Roden D. Extracellular potassium modulation of drug block of Ikr // Circulation. – 1996; 93:407–11. 32. Yang C. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease // Stroke. – 1998; 29:411–4. 33. Zhu Y., Auerbach A. K+-occupancy of the n-methyl-d-aspartate receptor channel probed by Mg2+-block // J. Gen. Physiol. – 2001; 117(3): 287–98.
How to prevent seizures
Measures aimed at eliminating and preventing the occurrence of seizures should include:
- giving up harmful addictions (smoking, excessive consumption of alcohol, coffee, tonic drinks); — sufficient daily fluid intake; — physical education to improve blood circulation; - enriching the body with vitamins and microelements (deficiencies in specific compounds can be identified using tests); - Night cramps can be prevented by a warm bath or shower.
To treat and prevent seizures, purchase a complex containing magnesium as a therapeutic measure. Even if there is no deficiency of this mineral in the body, it acts as an anticonvulsant element that reduces neuromuscular excitability.