| Prescription drug | |
| Release form | 100 or 150 mcg of active substance in a solution for subcutaneous administration of 0.5 ml, in a disposable 1 ml syringe made of colorless glass type I (Eur. Pharm.) with an automatic needle locking system. |
| Manufacturer | Vetter Pharma-Fertigung GmbH & Co. KG, Germany |
| Active substance | Corifollitropin alfa |
To learn more
Consultations are conducted by professional doctors who have significant experience in solving infertility problems.
Composition and release form
| Corifollitropin alfa | 100 or 150 mcg |
| Excipients: | |
| sodium citrate dihydrate | 3.68 mg |
| sucrose | 35.0 mg |
| L-methionine | 0.25 mg |
| polysorbate-20 | 0.10 mg |
| hydrochloric acid (or sodium hydroxide) | 0.1 M to pH 7.0 |
| water for injections | (extractable volume) up to 0.50 ml |
Elonva is available as a solution for subcutaneous administration in 0.5 ml syringes. The package contains 1 syringe, as this is a long-acting drug. There are two dosages - 100 mcg and 150 mcg.
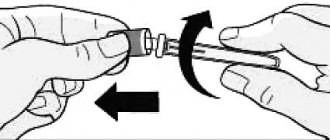
The syringe is made of transparent glass. It is equipped with an automatic needle locking system. On one side the syringe is closed with a cap. On the other side there is a piston with a rubber plug. The kit includes 1 sterile needle.
Elonva subcutaneous solution 150 mcg/0.5 ml 0.5 ml x1 syringe complete with needle
Marketing authorization holder: NV ORGANON (Netherlands)
Manufactured by: VETTER PHARMA-FERTIGUNG, GmbH &, Co.KG (Germany) Active substance: corifollitropin alfa Rec.INN registered by WHO
Dosage form
, Elonva®
Solution for subcutaneous administration 150 mcg/0.5 ml: syringes 1 ml 1 pc. with needlereg. No.: LP-001212 from 11/15/11 - Indefinitely
Release form, packaging and composition of Elonva®
The solution for subcutaneous administration is transparent, colorless to light yellow.
0.5 ml
corifollitropin alfa 150 mcg
Excipients: sodium citrate dihydrate - 3.68 mg, sucrose - 35 mg, L-methionine - 250 mcg, polysorbate 20 - 100 mcg, hydrochloric acid 0.1M or sodium hydroxide 0.1M - up to pH 7.0, water d/i (extractable volume) - up to 0.5 ml.
0.5 ml - disposable syringes with a volume of 1 ml made of colorless glass (1) with an automatic needle locking system, complete with a sterile needle (1) - plastic containers (1) - cardboard packs.
Clinical and pharmacological group: Recombinant human follicle-stimulating hormone Pharmaco-therapeutic group: Follicle-stimulating agent
pharmachologic effect
Follicle stimulating agent. Corifollitropin alpha is a recombinant DNA glycoprotein produced by Chinese hamster ovary cells. A long-acting follicle growth stimulator, its pharmacodynamic properties are comparable to recombinant FSH (rFSH), but has a significantly longer effect.
Corifollitropin alfa induces and maintains follicular growth for a week. An increase in the duration of follicle-stimulating activity was achieved by attaching the carboxy-terminal peptide of the beta subunit of human chorionic gonadotropin (hCG) to the beta chain of human FSH. Corifollitropin alfa does not have hCG and LH activity.
Pharmacokinetics
The pharmacokinetics of corifollitropin alfa were independent of dose over a wide dose range (7.5-240 mcg). The distribution, metabolism and elimination of corifollitropin alfa are similar to those of other gonadotropins such as FSH, hCG and LH.
After a single subcutaneous administration of corifollitropin alfa, Cmax in plasma was achieved after 44 hours (34-57 hours). Absolute bioavailability was 58% (48-70%). Corifollitropin alfa exposure is dependent on body weight. In clinical studies, plasma concentrations of corifollitropin alfa were similar following administration of corifollitropin alfa 100 mcg and 150 mcg to women weighing ≤60 kg and >.60 kg, respectively.
After absorption into the blood, corifollitropin alfa is distributed mainly to the ovaries and kidneys. At steady state, Vd and clearance are 9.2 l (6.5-13.1 l) and 0.13 l/h (0.10-0.18 l/h), respectively.
The metabolism of corifollitropin alfa primarily involves the kidneys. As a result of metabolism, pharmacologically inactive alpha and beta subunits (including the carboxy-terminal peptide) are formed, which are primarily excreted by the kidneys.
T1/2 of corifollitropin alfa is 69 hours (59-79 hours). Corifollitropin alfa is excreted primarily by the kidneys.
Indications of the active substances of the drug Elonva® Controlled ovarian stimulation in combination with GnRH antagonists to form multiple follicles in women participating in an assisted reproduction program.
Dosage regimen The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed.
It is administered subcutaneously (preferably under the skin of the abdomen) in accordance with special schemes.
Recommended doses have been established only for combined use with a GnRH antagonist.
Women weighing <.60 kg are given a single dose of 100 mcg.
Women weighing >.60 kg are given a single dose of 150 mcg.
Side effect
Determination of the frequency of adverse reactions: often (≥ 1%, < 10%), uncommon (≥ 0.1%, < 1%).
From the nervous system: often - headache, infrequently - dizziness.
From the digestive system: often - nausea, infrequently - abdominal pain, vomiting, diarrhea, constipation, bloating.
From the reproductive system: often - OHSS, pain and discomfort in the pelvic area, complaints from the mammary glands, infrequently - ovarian torsion. In addition, ectopic pregnancy, miscarriage and multiple pregnancy have been described and are considered complications of assisted reproduction methods.
General reactions: often - fatigue.
Contraindications for use
Tumors of the ovaries, breast, uterus, pituitary gland or hypothalamus, bleeding and spotting from the genital tract (not associated with menstruation) of unknown cause, primary ovarian failure, ovarian cysts or ovarian enlargement, history of OHSS, if the previous cycle of controlled stimulation ovaries led to the growth of more than 30 follicles to a size of at least 11 mm, identified by ultrasound, the number of basal antral follicles more than 20, identified by ultrasound, fibroid tumors of the uterus, in which the onset and further pregnancy is difficult, malformations of the reproductive organs, in which pregnancy is impossible, pregnancy, lactation (breastfeeding), hypersensitivity to corifollitropin alfa.
Use during pregnancy and breastfeeding
Contraindicated for use during pregnancy and lactation (breastfeeding).
After controlled stimulation of the ovaries with gonadotropins in clinical practice, no teratogenic effect was detected. Clinical data do not allow us to exclude the teratogenic effect of corifollitropin alfa if it is unintentionally administered during pregnancy.
In preclinical studies, no teratogenic effect of corifollitropin alfa was observed.
special instructions
Before starting treatment, the couple must be properly examined, a diagnosis of infertility made and possible contraindications taken into account. In particular, the patient should be examined for the presence of hypothyroidism, adrenal insufficiency, hyperprolactinemia and tumors of the pituitary gland or hypothalamus, and if detected, appropriate treatment should be prescribed.
It is not recommended to use corifollitropin alfa in combination with a GnRH agonist, or in women with polycystic ovary syndrome.
Corifollitropin alfa is intended for single subcutaneous administration only. Additional injections of this drug should not be given during the same cycle.
rFSH should not be administered during the first 7 days after administration of corifollitropin alfa.
In patients with renal insufficiency, the elimination of corifollitropin alfa may be impaired and therefore use in such patients is not recommended.
Careful monitoring of possible ovarian hyperstimulation is recommended in the first cycle of stimulation in patients with unspecified risk factors for OHSS.
When using all gonadotropin drugs, cases of multiple pregnancies and the birth of twins were observed. Before starting treatment, the woman and her partner should be informed about the possible risks for the mother (complications of pregnancy and childbirth) and newborns (low body weight). When treated with assisted reproductive technologies, the risk of multiple pregnancy mainly depends on the number of embryos transferred.
Women with infertility who are offered treatment with assisted reproductive technologies, especially in vitro fertilization (IVF), often have pathology of the fallopian tubes, which can lead to an increased risk of ectopic pregnancy. In this regard, an ultrasound examination should be performed in early pregnancy to confirm the presence of an intrauterine or ectopic pregnancy.
The incidence of congenital malformations after assisted reproductive technologies is slightly higher than after natural fertilization. This is associated with the individual characteristics of the parents (for example, the woman’s age, sperm counts) and the increased frequency of multiple pregnancies.
In women with risk factors for thromboembolic complications (history of thromboembolism, family history, obesity (body mass index > 30 kg/m2) or thrombophilia), treatment with gonadotropins may further increase this risk. In such cases, it is necessary to evaluate the risks and benefits of using gonadotropins. It should be noted that pregnancy itself increases the risk of thrombosis.
Impact on the ability to drive vehicles and machinery
Corfollitropin alfa may cause dizziness. Patients should be warned that if dizziness occurs, they should not drive vehicles or use complex equipment.
Compound
Elonva contains corifollitropin alfa. This is a recombinant hormone produced by hamster ovary cells. It is used as a replacement for follicle stimulating hormone (FSH), which is produced in the human body.
Elonva differs from FSH drugs in that it lasts much longer. Corifollitropin alfa is administered once a week at the initial stage of ovulation stimulation. Increased duration of action was achieved by attaching the carboxy-terminal peptide of the β-subunit of hCG. At the same time, Elonva does not have the clinical effects characteristic of human chorionic gonadotropin. The drug does not have luteinizing hormone (LH) activity.
Bioavailability
The absolute bioavailability of Elonv when administered subcutaneously averages 58%. Although in different patients it ranges from 48 to 70%. This means that on average 58% of the dose is absorbed into the systemic circulation after a subcutaneous injection of the drug.
The maximum concentration in the blood plasma of the active substance Elonva is achieved after 2 days. After administration, the drug accumulates to the greatest extent in the ovaries and kidneys. In the ovaries it acts by stimulating the maturation of follicles, and is excreted from the body through the kidneys.
The half-life of the drug is about 70 hours. In case of renal failure, Elonva is eliminated from the body more slowly. The liver takes virtually no part in the metabolism of corifollitropin-alpha.
Contraindications
Elonva has a number of contraindications, the presence of which should be taken into account before starting therapy. The drug is not prescribed for the following diseases and conditions:
- allergy to corifollitropin-alpha or any of the additional components of the drug;
- oncological formations of the reproductive system, which are localized in the ovaries, mammary gland, structures of the hypothalamic-pituitary system;
- uterine bleeding of unknown origin;
- primary ovarian failure;
- growth of 30 or more follicles with a diameter of 11 mm or more in the previous IVF cycle;
- ovarian hyperstimulation syndrome (OHSS) in one of the previous IVF cycles;
- the presence of 20 or more antral follicles in the ovaries on days 2-3 of the menstrual cycle;
- oncological formations (including benign ones) of the uterus and other diseases that make pregnancy impossible (increasing the risk of spontaneous abortion);
- malformations of the reproductive system that make the onset or development of pregnancy impossible;
- pregnancy;
- lactation;
- polycystic ovary syndrome;
- insufficiency of kidney function.
Elonva is not prescribed for malignant tumors, because the drug can stimulate the growth of cancerous tumors, thereby accelerating the progression of the disease.
If there is insufficient ovarian function, there is no point in prescribing the drug. Because the ovaries will not respond to corifollitropin-alpha. Under the influence of the drug, follicles will not ripen in them. Ovarian failure requires the use of IVF with donor oocytes. This is the only way to achieve pregnancy in this situation.
Most contraindications are associated with the risk of ovarian hyperstimulation syndrome. It occurs as a result of increased production of hormones and vasoactive substances under the influence of drug therapy. Extensive experience with IVF has allowed doctors to identify the main risk factors for OHSS in order to prevent this condition. Reproduction specialists predict the response to stimulation. Therefore, if the risk factors listed in the contraindications are present, Elonva is not prescribed. The main way to predict response to stimulation is to do an ultrasound at the beginning of the cycle. Previous experience in stimulating superovulation is also used.
For obvious reasons, Elonva is not used during pregnancy. Because this drug is used exclusively to ensure that this very pregnancy occurs. If you are already pregnant, you do not need to stimulate the maturation of eggs. In addition, animal studies have shown that corifollitropin alfa is toxic to the developing fetus.
Any contraindications to pregnancy are also contraindications to the use of Elonv. Because, as we just said, the only purpose of its use is to conceive a child. Pregnancy is contraindicated in all cases where it threatens the health and life of the mother, or if pregnancy is impossible for some reason.
Finally, the last contraindication is renal failure. This is due to the fact that the drug is excreted through the kidneys. If their function is insufficient, an increase in the time of exposure of Elonv to the woman’s body is observed. As a result, the risk of ovarian hyperstimulation increases significantly. Which, moreover, against the background of renal failure can be much more severe.
Elonva solution for subcutaneous administration 150 µg/0.5 ml syringe with needle
ATX Code:
G03GA09 (Corifollitropin alfa)
Active substance:
corifollitropin alfa
Release form, packaging and composition of the drug
The solution for subcutaneous administration is transparent, colorless to light yellow.
corifollitropin alfa - 100 mcg
Excipients: sodium citrate dihydrate - 3.68 mg, sucrose - 35 mg, L-methionine - 250 mcg, polysorbate 20 - 100 mcg, hydrochloric acid 0.1M or sodium hydroxide 0.1M - up to pH 7.0, water d/i (extractable volume) - up to 0.5 ml.
0.5 ml - disposable syringes with a volume of 1 ml made of colorless glass (1) with an automatic needle locking system, complete with a sterile needle (1) - plastic containers (1) - cardboard packs.
Clinical and pharmacological group:
Recombinant human follicle stimulating hormone
Pharmacotherapeutic group:
Follicle stimulating agent
pharmachologic effect
Follicle stimulating agent. Corifollitropin alpha is a recombinant DNA glycoprotein produced by Chinese hamster ovary cells. A long-acting follicle growth stimulator, its pharmacodynamic properties are comparable to recombinant FSH (rFSH), but has a significantly longer effect.
Corifollitropin alfa induces and maintains follicular growth for a week. An increase in the duration of follicle-stimulating activity was achieved by attaching the carboxy-terminal peptide of the beta subunit of human chorionic gonadotropin (hCG) to the beta chain of human FSH. Corifollitropin alfa does not have hCG and LH activity.
Pharmacokinetics
The pharmacokinetics of corifollitropin alfa were independent of dose over a wide dose range (7.5-240 mcg). The distribution, metabolism and elimination of corifollitropin alfa are similar to those of other gonadotropins such as FSH, hCG and LH.
After a single subcutaneous administration of corifollitropin alfa, Cmax in plasma was achieved after 44 hours (34-57 hours). Absolute bioavailability was 58% (48-70%). Corifollitropin alfa exposure is dependent on body weight. In clinical studies, plasma concentrations of corifollitropin alfa were similar following administration of corifollitropin alfa 100 mcg and 150 mcg to women weighing ≤60 kg and >60 kg, respectively.
After absorption into the blood, corifollitropin alfa is distributed mainly to the ovaries and kidneys. At steady state, Vd and clearance are 9.2 l (6.5-13.1 l) and 0.13 l/h (0.10-0.18 l/h), respectively.
The metabolism of corifollitropin alfa primarily involves the kidneys. As a result of metabolism, pharmacologically inactive alpha and beta subunits (including the carboxy-terminal peptide) are formed, which are primarily excreted by the kidneys.
T1/2 of corifollitropin alfa is 69 hours (59-79 hours). Corifollitropin alfa is excreted primarily by the kidneys.
Indications of the active substances of the drug
Controlled ovarian stimulation in combination with GnRH antagonists to produce multiple follicles in women participating in an assisted reproduction program.
Dosage regimen
The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed.
It is administered subcutaneously (preferably under the skin of the abdomen) in accordance with special schemes.
Recommended doses have been established only for combined use with a GnRH antagonist.
Women weighing <60 kg are given a single dose of 100 mcg.
Women weighing >60 kg are given a single dose of 150 mcg.
Side effect
Determination of the frequency of adverse reactions: often (≥ 1%, < 10%), uncommon (≥0.1%, < 1%).
From the nervous system: often - headache; infrequently - dizziness.
From the digestive system: often - nausea; uncommon - abdominal pain, vomiting, diarrhea, constipation, bloating.
From the reproductive system: often - OHSS, pain and discomfort in the pelvic area, complaints from the mammary glands; infrequently - ovarian torsion. In addition, ectopic pregnancy, miscarriage and multiple pregnancy have been described and are considered complications of assisted reproduction methods.
General reactions: often - fatigue.
Contraindications for use
Tumors of the ovaries, breast, uterus, pituitary gland or hypothalamus; bleeding and spotting from the genital tract (not related to menstruation) of unknown cause; primary ovarian failure; ovarian cysts or enlarged ovaries; history of OHSS; if the previous cycle of controlled ovarian stimulation resulted in the growth of more than 30 follicles to a size of at least 11 mm, detected by ultrasound; the number of basal antral follicles is more than 20, identified by ultrasound; fibroid tumors of the uterus, in which the onset and further pregnancy is difficult; malformations of the reproductive organs, in which pregnancy is impossible; pregnancy; lactation period (breastfeeding); hypersensitivity to corifollitropin alfa.
Use during pregnancy and breastfeeding
Contraindicated for use during pregnancy and lactation (breastfeeding).
After controlled stimulation of the ovaries with gonadotropins in clinical practice, no teratogenic effect was detected. Clinical data do not allow us to exclude the teratogenic effect of corifollitropin alfa if it is unintentionally administered during pregnancy.
In preclinical studies, no teratogenic effect of corifollitropin alfa was observed.
special instructions
Before starting treatment, the couple must be properly examined, a diagnosis of infertility made and possible contraindications taken into account. In particular, the patient should be examined for the presence of hypothyroidism, adrenal insufficiency, hyperprolactinemia and tumors of the pituitary gland or hypothalamus, and if detected, appropriate treatment should be prescribed.
It is not recommended to use corifollitropin alfa in combination with a GnRH agonist, or in women with polycystic ovary syndrome.
Corifollitropin alfa is intended for single subcutaneous administration only. Additional injections of this drug should not be given during the same cycle.
rFSH should not be administered during the first 7 days after administration of corifollitropin alfa.
In patients with renal insufficiency, the elimination of corifollitropin alfa may be impaired and therefore use in such patients is not recommended.
Careful monitoring of possible ovarian hyperstimulation is recommended in the first cycle of stimulation in patients with unspecified risk factors for OHSS.
When using all gonadotropin drugs, cases of multiple pregnancies and the birth of twins were observed. Before starting treatment, the woman and her partner should be informed about the possible risks for the mother (complications of pregnancy and childbirth) and newborns (low body weight). When treated with assisted reproductive technologies, the risk of multiple pregnancy mainly depends on the number of embryos transferred.
Women with infertility who are offered treatment with assisted reproductive technologies, especially in vitro fertilization (IVF), often have pathology of the fallopian tubes, which can lead to an increased risk of ectopic pregnancy. In this regard, an ultrasound examination should be performed in early pregnancy to confirm the presence of an intrauterine or ectopic pregnancy.
The incidence of congenital malformations after assisted reproductive technologies is slightly higher than after natural fertilization. This is associated with the individual characteristics of the parents (for example, the woman’s age, sperm counts) and the increased frequency of multiple pregnancies.
In women with risk factors for thromboembolic complications (history of thromboembolism, family history, obesity (body mass index >30 kg/m2) or thrombophilia), treatment with gonadotropins may further increase this risk. In such cases, it is necessary to evaluate the risks and benefits of using gonadotropins. It should be noted that pregnancy itself increases the risk of thrombosis.
Impact on the ability to drive vehicles and operate machinery
Corfollitropin alfa may cause dizziness. Patients should be warned that if dizziness occurs, they should not drive vehicles or use complex equipment.
Use of the drug in an IVF cycle
Elonva is prescribed by a fertility specialist. The use of the drug is allowed only if the solution inside the syringe is transparent.
The dosage depends on the woman’s age and body weight. All are available in 2 dosages - 100 and 150 mcg. A smaller dose is administered to patients aged 35 years or less, with a body weight of 60 kilograms or less. Dosages of 150 mcg are used in the following cases:
- body weight above 60 kg (regardless of age);
- body weight more than 50 kg, aged 36 years or older.
Stimulation begins in the first phase of the menstrual cycle. On the first day, the drug is administered subcutaneously. Typically the injection is given in the abdomen. This allows for better bioavailability of the drug.
Starting from the 5th or 6th day of the cycle, GnHR antagonists (gonadotropin releasing hormone) are administered. When to administer it depends on the number of growing follicles and their size. These parameters are determined during transvaginal ultrasound. The level of hormones in the blood, including estradiol, must be monitored. The administration of GnGR is necessary to prevent a premature increase in the concentration of luteinizing hormone in the blood.
Elonva is valid for 7 days. Starting from the 8th day of stimulation, treatment with FSH drugs should be continued. They have the same range of clinical effects. But they have a shorter duration of action.
FSH is prescribed once a day. The standard dosage is 150 IU. But it can be increased or decreased at the discretion of the doctor. Because different women's ovaries react differently to stimulation. The answer may be too weak or, on the contrary, too strong. With a weak response, the follicles do not mature to the required size. With a strong response, the risk of ovarian hyperstimulation syndrome increases. Therefore, to avoid these negative consequences, folliculogenesis is monitored using ultrasound. In this way, the doctor evaluates the response to stimulation. If it is weak, the dose of FSH is increased, and if it is strong, it is reduced. The drug is continued until 3 or more follicles with a diameter of 17 mm are detected in the ovaries.
For the average woman, follicle maturation occurs on day 9 of stimulation. That is, only 2 injections of FSH are required. But in some situations, longer therapy is required. Stimulation can sometimes last up to 18 days.
After the doctor detects 3 follicles measuring 17 mm or more on an ultrasound, the hCG hormone is prescribed. It is administered once in a dose of 5 or 10 thousand IU. It is required for the final maturation of eggs. HCG can be administered on the same day or the next.
Subscribe to our newsletter
Subscription benefits
I accept the terms of the User Agreement and consent to the processing of my personal data
Using Elonva for ovarian stimulation
Elonva
is a drug that is used in IVF protocols at the stage of ovulation stimulation. The purpose of its purpose is the formation of several follicles at once in a woman’s ovaries for the purpose of subsequent extraction of eggs from them.
This is a drug of the latest generation, which is increasingly used by doctors at reproductive centers in the country. The first pregnancy in Russia after an IVF protocol using Elonv was obtained at our VitroClinic.
Make an appointment
IVF drug Elonva
Elonva is a recombinant follicle-stimulating hormone. The active substance is corifollitropin alfa. Available in solution for subcutaneous administration. Release form - a syringe containing 100 or 150 mcg of active substance.
Compared to drugs of previous generations, it provides a more stable and long-term stimulation of the process of maturation of follicles in the ovaries. It is valid for a week. Thus, one Elonv injection can replace daily FSH injections for 7 days.
A single dose depends on the woman’s body weight. These doses are used when Elonva is co-administered with gonadotropin-releasing hormone (GnRH) antagonists. The drug is not used with GnRH agonists.
It was at VitroClinic that the first one was received! pregnancy in Russia after an IVF protocol using this drug Elonva.
IVF protocol with Elonva
The drug Elonva is prescribed only by a reproductologist who controls the entire process of stimulating ovulation during in vitro fertilization. It is administered once, subcutaneously, on the second or third day of the menstrual cycle.
On days 5-6 of stimulation, GnRH antagonists (for example, Orgalutran or Cetrotide) are administered. The optimal day is determined by the doctor based on measuring the level of estradiol in the blood, determining the size and number of maturing follicles.
On the 8th day of stimulation, daily injections of rFSH (for example, Gonal or Puregon) are prescribed. The dose is determined by the doctor individually. Typically, with a normal ovarian response, it is 150 IU. In some cases, if there is a threat of hyperstimulation, the dose may be reduced.
Starting from 5-6 days after the introduction of rFSH into the stimulation regimen, the doctor performs an ultrasound every day, monitoring the growth of follicles. Once they reach the desired size (17-18 mm), rFSH injections are stopped and a trigger dose of hCG (for example, Pregnil, Horagon or Ovitrel) is administered. This usually happens on the 9-10th day of stimulation.
Doctors keep up with the times, and therefore actively prescribe Elonva to women as part of the IVF protocol. Such stimulation schemes are much more patient-friendly and psychologically easier to tolerate, because the drug has a long-lasting effect and does not need to be administered every day.
You can buy Elonva at a pharmacy. This drug is always available here.
Side effects
Side effects are sometimes observed when using Elonva. All processes occurring in the body are completely reversible. Therefore, the occurrence of most side effects is not a reason to stop treatment. Here are the most common side effects that occur when using the drug:
- feeling of discomfort in the lower abdomen;
- headache;
- lower abdominal pain;
- ovarian hyperstimulation syndrome;
- nausea;
- painful or other unpleasant sensations in the mammary glands;
- increased fatigue.
The listed side effects occur with a frequency of 1.2 to 5.5%. All other side effects are recorded with a frequency of less than 1%. These include:
- lability of emotional state;
- backache;
- vegetative-vascular disorders;
- dyspepsia (flatulence, abdominal pain, vomiting);
- premature ovulation.
When superovulation is stimulated by Elonva, as well as any other hormonal drugs, the risk of multiple and ectopic pregnancy slightly increases.
Precautionary measures
- Do not exceed the dose higher than recommended by the manufacturer. Because this is highly likely to lead to the development of ovarian hyperstimulation syndrome. If, according to ultrasound data, the likelihood of this condition is assessed as high, then after one injection of Elonva, FSH stimulation is not prescribed from the 8th day.
- Before stimulation it is necessary to be examined. A woman must undergo hormone tests. She undergoes a pelvic ultrasound and hysterosalpingography to check the patency of the fallopian tubes. If the tubes are blocked, there is no point in stimulation, because pregnancy will be impossible. An ultrasound may reveal malformations or acquired diseases that are incompatible with pregnancy. Hormone tests may reveal adrenal insufficiency, thyroid insufficiency, or elevated prolactin levels. In these cases, treatment is required to normalize hormonal levels. Only after normalization of test results can stimulation and in vitro fertilization be carried out.
- FSH preparations should not be administered during treatment with Elonva earlier than a week after the injection. Elonva has a long-lasting effect. The drug takes a long time to clear from the blood. Therefore, additional administration of FSH before the 8th day of stimulation can lead to an overdose and ovarian hyperstimulation syndrome.
- Only one Elonv injection is given per cycle. This allows you to better control the cycle and prevent the development of OHSS. Elonva stimulates follicle maturation within 7 days. At the same time, the total duration of stimulation is usually 9 or 10 days. Thus, 2 administered doses of the drug are highly likely to be excessive. Therefore, treatment from day 8 continues only with FSH drugs.
- Elonva is administered subcutaneously. Other methods of administration are not used. Because this may lead to a change in the bioavailability of the drug. Accordingly, you will not get the effect you expect.
- Elonva should not be used with GnRH agonists. Because this combination is not enough research. At the moment there is no exact data on how safe it is for a woman’s body.
Elonva and ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome develops when there is an overdose of Elonv or the ovaries respond too strongly to stimulation with normal doses of the drug due to the peculiarities of the functioning of the woman’s reproductive system. With OHSS, the ovaries increase in volume. Usually the pathology occurs in a mild or moderate form. The woman may develop diarrhea and abdominal pain.
Very rarely the disease is severe. In this case, hospitalization is required. Clinical manifestations may include:
- dyspnea;
- decrease in daily diuresis;
- severe abdominal pain;
- accumulation of fluid in the abdominal cavity, which is manifested by an enlarged abdomen.
In severe forms of OHSS, cysts may appear in the ovaries, and thromboembolic complications sometimes develop.
Hyperstimulation syndrome after using Elonv can be early or late. Early develops in the first 10 days after injection of the drug. Late – more than 10 days later. Early is due to an increased response of the ovaries, and late is due to hormonal changes during pregnancy. It flows more severely.
Diagnostics for the prevention and timely detection of OHSS are carried out continuously throughout the entire period of stimulation plus 2 weeks after the administration of hCG. The woman is examined especially carefully during the first cycle. Because it is not yet known how the ovaries react to the drugs. In the future, the doctor identifies the main risk factors for OHSS and takes the necessary measures to prevent it.
The risk of OHSS is considered high if 18 or more follicles measuring 11 millimeters or more are detected. If in the first days of the cycle 30 or more antral follicles are detected, then the use of Elonv is not recommended. In this case, there is a high risk of OHSS.
To reduce the likelihood of ovarian hyperstimulation syndrome, your doctor may take the following measures:
- stop using gonadotropins (do not prescribe FSH from the 8th day of stimulation, limiting yourself to 1 injection of Elonv);
- introduce hCG at a dose of 5000, not 10,000 IU;
- do not administer hCG at all;
- cancel embryo transfer so that late OHSS does not develop (it develops only during pregnancy).
If the IVF program is suspended, the embryos are frozen. Then they can be transferred to the next cycle.
Instructions for use ELONVA
Do not use Elonva if the solution is not clear.
Before starting treatment, it is necessary to evaluate existing and suspected contraindications for pregnancy. In particular, the woman should be evaluated for hypothyroidism, adrenal insufficiency, hyperprolactinemia, hypothalamic and pituitary tumors, and appropriate treatment should be prescribed.
Elonva is intended for single subcutaneous administration only. Additional injections should not be given during the same cycle.
During the first 7 days after administration of the drug, rFSH should not be administered.
In patients with renal failure, the elimination of corifollitropin alfa may be impaired. In such women, the use of the drug is not recommended.
Experience with Elonva in combination with a gonadotropin-releasing hormone agonist is limited. The results of a small uncontrolled study indicate a more pronounced ovarian response compared with that of a combination of the drug with a gonadotropin-releasing hormone antagonist. In this regard, it is not recommended to use Elonva in combination with a gonadotropin-releasing hormone agonist.
Elonva has not been studied in women with polycystic ovary syndrome. In such cases, the use of the drug is not recommended.
After administration of the drug, the ovarian response was more pronounced than after daily injections of rFSH. Therefore, patients with known risk factors for a “strong” ovarian response may be particularly predisposed to developing ovarian hyperstimulation syndrome during or after drug administration. If this is the first cycle of ovarian stimulation and not all risk factors are known, careful monitoring of possible ovarian hyperstimulation is recommended.
Ovarian hyperstimulation syndrome (OHSS)
OHSS is different from uncomplicated ovarian enlargement. Clinical signs and symptoms of mild to moderate OHSS may include:
- abdominal pain, nausea, diarrhea, ovarian enlargement (slight to moderate), ovarian cyst. Severe OHSS can be life-threatening. Clinical signs and symptoms of severe OHSS include large ovarian cysts (prone to rupture), acute abdominal pain, ascites, pleural effusion, hydrothorax, shortness of breath, oliguria, hematological abnormalities, and weight gain. In rare cases, the development of OHSS can lead to venous or arterial thromboembolism.
Symptoms and signs of OHSS occur under the influence of human chorionic gonadotropin (hCG) or pregnancy (endogenous hCG). Early ovarian hyperstimulation syndrome usually develops within 10 days after hCG administration and may be accompanied by an excessive ovarian response to gonadotropin stimulation. Common early ovarian hyperstimulation syndrome goes away on its own after the onset of menstruation. Late ovarian hyperstimulation syndrome develops more than 10 days after hCG administration as a result of a (multiple) pregnancy. Given the risk of developing ovarian hyperstimulation syndrome, patients should be observed for at least 2 weeks after hCG administration.
To minimize the risk of developing ovarian hyperstimulation syndrome, ultrasound monitoring of growing follicles and/or serum estradiol levels should be performed prior to treatment and regularly during treatment. In assisted reproductive technology programs, the risk of developing ovarian hyperstimulation syndrome is increased if there are 18 or more follicles with a diameter of 11 mm or more. If the total number of follicles is ≥30, hCG should not be administered.
Depending on the response of the ovaries, the following measures can be taken to prevent ovarian hyperstimulation syndrome:
- postpone further stimulation with gonadotropin for a maximum of 3 days;
- delay induction of final oocyte maturation by hCG until estradiol levels stabilize or decrease;
- take hCG in a dose of less than 10,000 IU to induce final oocyte maturation, for example, 5,000 IU of hCG or 250 μg of rHCG, which is equivalent to approximately 6,500 IU;
- freeze all embryos for further implantation;
- do not administer hCG and stop the treatment cycle.
To support the luteal phase, hCG should be avoided.
To minimize the risk of developing ovarian hyperstimulation syndrome, it is necessary to adhere to the recommended dose and regimen of the drug and carefully monitor the ovarian response.
When using all gonadotropin preparations, cases of multiple pregnancies and the birth of several newborns were observed. Before starting treatment, the woman and her partner should be informed of the possible risks to the mother (complications of pregnancy and childbirth) and the newborn (low body weight). When treated with assisted reproductive technologies, the risk of multiple pregnancy mainly depends on the number of embryos transferred.
Infertile women who are offered treatment with assisted reproductive technologies (especially in vitro fertilization - IVF) often experience fallopian tube pathology, which can lead to an increased risk of ectopic pregnancy. In this regard, an ultrasound examination should be performed in early pregnancy to confirm a uterine pregnancy and exclude an ectopic pregnancy.
The incidence of congenital defects after assisted reproductive technologies is slightly higher than after natural fertilization. This is associated with the characteristics of the parents (for example, the woman’s age, sperm properties) and the increased frequency of multiple pregnancies.
Cases of the development of tumors of the ovaries and other organs of the reproductive system have been described in women receiving various treatment regimens for infertility. It has not been established whether treatment with gonadotropins can cause tumors in infertile women.
In women with risk factors for thromboembolic complications, such as a history of thromboembolism, family history, obesity (body mass index >30 kg/m2) or thrombophilia, treatment with gonadotropins may further increase this risk. In such cases, the benefits and risks of using gonadotropins should be weighed. It should be noted that pregnancy itself increases the risk of developing thrombosis.
The ability to influence the speed of reactions when driving vehicles or working with other mechanisms
No studies have been carried out on the effect of the drug on the rate of reactions when driving vehicles or operating other mechanisms. Elonva may cause dizziness. If a woman feels dizzy, she is not recommended to drive a vehicle or operate other machinery.
Other complications
While using Elonva, some other complications associated with this drug are possible, although very rare. Among them:
- ovarian torsion;
- multiple births;
- ectopic pregnancy;
- vascular complications.
The likelihood of ovarian torsion increases due to a significant increase in its size. The risk increases with:
- pregnancy as a result of assisted reproductive technologies;
- development of ovarian hyperstimulation syndrome;
- previous operations on the pelvic organs;
- existing ovarian cysts.
When using ART with Elonva or any other gonadotropins, the risk of multiple pregnancy increases. How high it will be depends on the number of embryos transferred.
After ART, the risk of ectopic pregnancy always increases, including after using Elonv. In this regard, all women who become pregnant in this way undergo an ultrasound scan at an early stage of gestation. It allows not only to reliably confirm a developing pregnancy, but also to find out the location of the fertilized egg.
Very rarely (single cases have been reported) treatment with gonadotropins is accompanied by the formation of blood clots inside the vessels. This is fraught with thromboembolism with decreased blood circulation in the lower extremities or some organs. Therefore, Elonva is used with caution when:
- obesity;
- varicose veins of the lower extremities;
- thrombophilia.
Pregnancy itself is also a factor that increases the likelihood of thrombosis. Therefore, before starting treatment, a woman takes a coagulogram. This is a blood clotting test. There are medications that can reduce the risk of blood clots. They are used in women with risk factors for vascular complications.
Elonva®
Diagnosis of infertility before treatment
- Before starting treatment, the couple should be properly examined, in particular, the patient should be examined for the presence of hypothyroidism, adrenal insufficiency, hyperprolactinemia and tumors of the pituitary gland or hypothalamus. If they are detected, appropriate treatment should be prescribed. Before starting treatment with Elonva®, you should also take into account diseases that are contraindications to pregnancy.
Dosing regimen during nickel stimulation
— Elonva® is intended for single subcutaneous administration only. Additional injections of Elonva® should not be prescribed during this cycle (see section "Dosage and Administration").
— After administration of Elonva®, do not additionally administer drugs containing FSH until the 8th day of stimulation (see section “Dosage and Administration”).
Kidney failure
- In patients with renal impairment, the elimination rate of corifollitropin alfa may be reduced. The use of the drug in such patients is contraindicated.
Use with a GnRH agonist is not recommended
Experience with Elonva® in combination with a GnRH agonist is limited. In this regard, the use of Elonva® in combination with a GnRH agonist is not recommended.
Ovarian hyperstimulation syndrome (OHSS)
— OHSS is a condition distinct from uncomplicated ovarian enlargement. Clinical manifestations of mild to moderate OHSS include abdominal pain, nausea, diarrhea, mild to moderate ovarian enlargement, and ovarian cysts. Severe OHSS may be life-threatening. Clinical manifestations of severe OHSS include large ovarian cysts, acute abdominal pain, aspitis, pleural effusion, hydrothorax, shortness of breath, and oliguria. changes in the blood and weight gain. In rare cases, venous or arterial thromboembolism may develop. In patients with OHSS, there were also cases of granular changes in liver function indicators, indicating liver dysfunction, which were not accompanied or were accompanied by morphological changes identified during liver biopsy. The development of OHSS may be a consequence of the use of hCG or pregnancy (endogenous hCG). Early OHSS usually develops within 10 days after hCG administration and may be associated with an excessive ovarian response to gonadotropin stimulation. Late OHSS develops more than 10 days after hCG administration due to hormonal changes during pregnancy. Considering the risk of developing OHSS. Patients should be observed for at least 2 weeks after hCG administration. Women with known risk factors for increased ovarian response are particularly likely to develop OHSS during or after use of gonadotropins, including Elonva®. During the first cycle of ovarian stimulation, when risk factors are only partially known, careful monitoring of early symptoms of OHSS is required.
To reduce the risk of developing OHSS, ultrasound monitoring of growing follicles should be performed before treatment and regularly during treatment. It is also necessary to simultaneously determine the concentration of estradiol in the blood plasma. In ART programs, the risk of developing OHSS is increased in the presence of 18 or more follicles with a diameter >11 mm. If the total number of follicles is 30 or more, hCG should not be administered.
Measures to reduce the risk of developing OHSS depending on the severity of the response
ovaries
— Refrain from further stimulation with gonadotropin for a maximum period of 3 days.
— Cancellation of hCG and termination of the therapeutic cycle.
- To induce final maturation of the oocyte, use hCG isolated from urine in a dose of less than 10,000 IU (for example, 5000 IU hCG isolated from urine) or 250 μg of recombinant choriogonadotropin alpha, which is equivalent to approximately 6500 IU hCG isolated from urine.
— Cancellation of embryo transfer followed by its cryopreservation.
— Cancellation of hCG to support the luteal phase.
To reduce the risk of developing OHSS, it is necessary to use the recommended doses and
dosage regimens for Elonva® and carefully monitor ovarian response. At
development of OHSS should follow standard approaches and prescribe appropriate
treatment.
Ovarian torsion
— After treatment with gonadotropins. including Elonva®, cases of ovarian torsion have been observed. Ovarian torsion may be associated with other conditions such as OHSS, pregnancy, previous abdominal surgery, past ovarian torsion, and current or history of ovarian cysts. Early diagnosis and immediate treatment of torsion can reduce damage to the ovary associated with decreased blood supply to the ovary.
Multiple pregnancy
— With the use of all gonadotropin drugs, including Elonva®, cases of multiple pregnancies and the birth of twins have been observed. Before starting treatment, the woman and her partner should be informed about the possible risks for the mother (complications of pregnancy and childbirth) and newborns (low body weight). When treated using ART methods, the risk of multiple pregnancy mainly depends on the number of embryos transferred.
Ectopic pregnancy
— Women with infertility who are treated with ART methods have an increased risk of ectopic pregnancy. In this regard, an ultrasound examination should be performed in early pregnancy to confirm the presence of intrauterine pregnancy and exclude ectopic pregnancy.
Congenital malformations
— The frequency of congenital malformations after the use of ART is slightly higher than after natural fertilization. This is associated with the individual characteristics of the parents (for example, the woman’s age, sperm characteristics) and the increased frequency of multiple pregnancies.
Tumors of the ovaries and other organs of the reproductive system
— In women who received various treatment regimens for infertility, cases of the development of both benign and malignant tumors of the ovaries and other organs of the reproductive system have been described. It has not been established whether treatment with gonadotropins may increase the risk of developing these tumors in infertile women.
Vascular complications
Thromboembolic complications, associated or not associated with OHSS. observed during treatment with gonadotropins, including Elonva®. Intravascular thrombosis, which can develop in the veins or arteries, can lead to decreased blood supply to the extremities or vital organs. In women with risk factors for thromboembolic complications (history of thromboembolism, family history, obesity or thrombophilia), treatment with gonadotropins may further increase this risk. In such cases, it is necessary to evaluate the risks and benefits of using gonadotropins. It should be noted that pregnancy itself increases the risk of thrombosis.