Biosulin N, 100 IU/ml, suspension for subcutaneous administration, 10 ml, 1 pc.
Instructions to be given to the patient
Injection technique when using insulin in vials
Do not use Biosulin® N if, when mixing the contents of the bottle according to the instructions for use, the insulin does not become uniformly white and cloudy.
Do not use Biosulin® N if it contains flakes after mixing.
Do not use Biosulin® N if hard white particles are stuck to the bottom or walls of the bottle, creating a “frost pattern” effect.
If the patient is using only one type of insulin
1. Disinfect the rubber membrane of the bottle.
2. Fill the syringe with air in a volume corresponding to the required dose of insulin. Inject air into the insulin vial.
3. Turn the bottle with the syringe upside down and draw the required dose of insulin into the syringe. Remove the needle from the vial and remove air from the syringe. Check that your insulin dose is correct.
4. Inject immediately.
If a patient needs to mix two types of insulin
1. Disinfect the rubber membranes of the bottles.
2. Immediately before drawing, roll the vial of long-acting insulin (“cloudy”) between your palms until the insulin becomes uniformly white and cloudy.
3. Fill the syringe with air in a volume corresponding to the dose of “cloudy” insulin. Inject air into the vial with “cloudy” insulin and remove the needle from the vial (“do not draw cloudy” insulin at this stage yet).
4. Fill the syringe with air in a volume corresponding to the dose of short-acting (“transparent”) insulin. Inject air into the vial of clear insulin. Turn the bottle with the syringe upside down and draw the required dose of “transparent” insulin. Remove the needle and remove air from the syringe. Check the correct dose.
5. Insert the needle into the vial with “cloudy” insulin, turn the vial with the syringe upside down and draw the required dose of insulin. Remove air from the syringe and check that the dose is correct. Inject the collected insulin mixture immediately.
6. Always draw insulins in the same sequence described above.
Injection technique when using insulin cartridges
The cartridge with the drug Biosulin® N is intended for use with the BiomaticPen® syringe pen. The patient should be warned about the need to carefully follow the instructions in the instructions for using the syringe pen for administering insulin.
Before use, make sure that there is no damage (for example, cracks) on the cartridge with Biosulin® N. Do not use the cartridge if there is any visible damage. Do not use Biosulin® N if, when mixing the contents of the cartridge according to the instructions for use, the insulin does not become uniformly white and cloudy.
Do not use Biosulin® N if it contains flakes after mixing. Do not use Biosulin® N if hard white particles are stuck to the bottom or walls of the cartridge, creating a “frost pattern” effect.
After the cartridge is inserted into the pen, a colored stripe should be visible through the window of the cartridge holder.
Before placing the cartridge into the pen, you should turn the cartridge up and down so that the glass ball moves from end to end of the cartridge. This procedure should be repeated at least 10 times until all the liquid becomes white and uniformly cloudy. Immediately after this you need to give an injection.
If the cartridge is already inside the pen, you should turn it with the cartridge inside up and down at least 10 times. This procedure must be repeated before each injection.
After injection, the needle should remain under the skin for at least 6 seconds. You should keep the button pressed until the needle is completely removed from under the skin, thus ensuring the correct dose is administered and limiting the possibility of blood or lymph getting into the needle or into the insulin cartridge.
The cartridge with Biosulin® N is intended for individual use only and cannot be refilled.
Injection procedure
Use two fingers to gather a fold of skin, insert the needle into the base of the fold at an angle of about 45° and inject insulin under the skin.
After injection, the needle should remain under the needle for at least 6 seconds to ensure that the insulin is completely injected.
If blood appears at the injection site after removing the needle, lightly press the injection site with a swab moistened with a disinfectant solution (for example, alcohol).
It is necessary to change injection sites.
INSTRUCTIONS FOR INJECTION OF THE MEDICINE Biosulin® N, suspension for subcutaneous administration 100 IU/ml USING A SYRINGE PEN BiomaticPen®2
(single use for multiple injections)
Appearance and parts of the BiomaticPen®2 syringe pen
1 — cap, 2 — body, 3 — dosage indicator window, 4 — dose setting ring, 5 — start button
Before use, make sure that there is no damage (for example, cracks) on the cartridge with Biosulin® N. Do not use a pen with a cartridge if there is any visible damage. Do not use the drug if, when mixing the contents of the cartridge according to the instructions for use, the insulin does not become uniformly white and cloudy.
Do not use a syringe pen with Biosulin® N if there are flakes in the cartridge after mixing. Do not use a syringe pen with Biosulin® N if hard white particles are stuck to the bottom or walls of the cartridge, creating a “frosty pattern” effect.
Ensuring asepsis during injection
Before giving the injection, you must wash your hands. It is very important that your hands and all equipment needed for injection are clean. Select the injection site. Wipe the skin at the injection site with an alcohol wipe only after the insulin dose has been set in the syringe pen. Before injection, allow the alcohol to dry at the injection site.
| Preparation |
| 1. Before using the BiomaticPen®2 syringe pen with Biosulin® N for the first time, roll the syringe pen between your palms 10 times. Then turn the syringe pen up and down 10 times so that the glass ball moves from end to end of the cartridge (position 1–2). These manipulations must be repeated until the suspension becomes uniformly white and cloudy. Immediately after this you need to give an injection. Before each injection: Turn the pen up and down 10 times so that the glass ball moves from end to end of the cartridge (position 1-2). Repeat this manipulation until the suspension becomes uniformly white and cloudy. Immediately after this you need to give an injection |
| Attaching a needle to a syringe pen |
| 2. Before use, remove the protective cap from the syringe pen. Disinfect the rubber membrane of the cartridge using an alcohol wipe or medical swab soaked in alcohol to prevent the entry of microorganisms. |
| 3. Remove the protective film from the outer protective cap of the needle. Attention! For each injection, use a new, sterile pen needle (with the protective film intact)! Read the instructions for using the pen needle! |
| 4. Carefully and tightly screw the needle onto the threads of the cartridge holder tip until it stops. |
| 5. Remove the outer needle protective cap and save it for removal and disposal of the used needle. |
| 6. Remove and discard the inner needle protective cap. Attention! The needle of the syringe pen is sterile! Don't touch her! Use a new needle for each injection to prevent infection. To avoid accidental needle sticks, never put the inner cap back on the needle. |
| Preparing a syringe pen for injection |
| 7. Turn the dose setting ring clockwise to set the test dose to 2 units. |
| 8. Install the BiomaticPen®2 syringe pen with the working end up and gently tap the cartridge holder so that all the air contained in the cartridge rises to the top. |
| 9. While holding the pen with the needle up, press the start button all the way. The dosage indicator will return to zero (position “0”). |
| 10. A few drops of the drug should appear at the end of the needle. If this does not happen, the operation (steps 7–9) should be repeated. If no drops appear, use a new needle (the needle may become clogged). Attention! To ensure that the dose is complete, you should always check that a drop of liquid comes out of the needle before each dose. |
| Setting the required dose of the drug |
| 11. Make sure the dosage indicator is in the “0” position. Set the number of units required to inject the drug by turning the dose setting ring clockwise (see the table for converting the BiomaticPen®2 syringe pen dosage indicator readings into the drug dose). The dose can be adjusted by rotating the dose setting ring in any direction until the correct dose is set against the dosage indicator. Attention! When rotating the dose setting ring, be careful not to accidentally press the start button to avoid releasing the dose of the drug. Safety limiter. The dose of the drug, which is installed on the BiomaticPen®2 syringe pen, can be limited by the amount remaining in the cartridge. If the amount of drug remaining in the cartridge is not sufficient for the required dose, the dose setting ring will not turn further in the clockwise direction. Throw away the pen or administer the remaining dose units and use a new pen to complete the required dose. |
| Carrying out the injection |
| 12. The injection of the drug should be carried out in accordance with the recommendations of the attending physician. To administer the set dose of the drug, press the start button all the way and hold it down throughout the entire administration process until the value “0” appears opposite the dosage indicator. A “0” value in the dosage indicator window means that you have administered your dose completely. Note: You can interrupt the injection of the drug by releasing the trigger button. The amount of drug that was not administered will be displayed in the dosage indicator window and can be additionally entered by pressing the start button again. |
| 13. After injection, the needle should remain under the skin for at least 6 seconds. You should keep the button pressed until the needle is completely removed from under the skin, thus ensuring the correct dose is administered and limiting the possibility of blood or lymph getting into the needle or into the insulin cartridge. Attention! Failure to follow these steps may result in the wrong dose being administered. If insulin continues to leak from the needle after an injection, hold the needle longer for subsequent injections. |
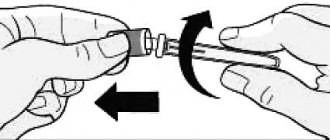
| 14. After removing the needle from under the skin, carefully place the outer protective cap on the needle of the syringe pen. |
| 15. Disconnect the needle by turning it counterclockwise and dispose of it properly. Attention! Strictly follow safety precautions to avoid accidental needlestick injury and possible transmission of infectious diseases. |
| 16. Close the syringe pen with the protective cap after each use to prevent direct sunlight and dust from entering the cartridge. |
Additional Information
Sound and tactile signals
During operation, the BiomaticPen®2 syringe pen produces the following sound and tactile signals:
— setting the required dose
When rotating the dose setting ring, a certain physical resistance is felt and clicks are heard as each dose unit is dialed.
- injection
The process of administering a drug from a BiomaticPen®2 syringe pen is accompanied by a sound signal (ratchet), which stops when the drug is completely introduced (to the value “0” in the dosage indicator window).
Rules for storage, use and disposal
The syringe pen is intended for individual use and cannot be used by several people.
Handle the BiomaticPen®2 syringe pen carefully.
Do not allow dust or moisture to get into the BiomaticPen®2 syringe pen.
After each use, close the syringe pen with the cap. Always keep the BiomaticPen®2 syringe pen in its individual packaging without a needle.
Store the BiomaticPen®2 syringe pen following the instructions for storing the medicinal product.
You can clean the BiomaticPen®2 syringe pen with a damp cloth. Do not use alcohol, solvents or other cleaning agents.
Never immerse the BiomaticPen®2 syringe pen in water, as this may damage it.
Warnings
Use the BiomaticPen®2 syringe pen only with pen-compatible needles recommended by your doctor.
Biosulin N should be used only as part of therapy prescribed by your doctor and in the dosage prescribed for you. Any changes should be made under the supervision of a physician.
If you have questions regarding needle length, consult your doctor or healthcare team.
Do not expose the BiomaticPen®2 syringe pen to extreme temperatures, do not leave it in direct sunlight or in the cold (for example, in the freezer). Keep the BiomaticPen®2 pen and pen needles out of the reach of children and others who are not familiar with proper handling techniques. In cases of unintentional administration of the drug or injury from a needle stick, you should immediately seek medical help!
Pen needles should only be used by one person to prevent the transmission of infectious diseases.
Use a new pen needle for each injection to ensure sterility. Remove the pen needle after injecting to prevent drug leakage, air ingress, and possible clogging of the pen needle. Dispose of used pen needles together with their protective cap, following the manufacturer's instructions, so that they do not cause harm to others.
Never use the BiomaticPen®2 syringe pen if you have any doubts about its correct operation.
Disposal rules
The BiomaticPen®2 syringe pen does not contain components that are hazardous to the environment and can be disposed of with normal household waste.
The used BiomaticPen®2 syringe pen should be disposed of only with the needle disconnected.
Table for converting the dosage indicator readings of the BiomaticPen®2 syringe pen into the dose of the drug Biosulin® N, suspension for subcutaneous administration 100 IU/ml:
| Dosage indicator indications | Dose of Biosulin® N, ME |
| 1. | 1. |
| 2. | 2. |
| 3. | 3. |
| 4. | 4. |
| 5. | 5. |
| 6. | 6. |
| 7. | 7. |
| 8. | 8. |
| 9. | 9. |
| 10. | 10. |
| 11. | 11. |
| 12. | 12. |
| 13. | 13. |
| 14. | 14. |
| 15. | 15. |
| 16. | 16. |
| 17. | 17. |
| 18. | 18. |
| 19. | 19. |
| 20. | 20. |
| 21. | 21. |
| 22. | 22. |
| 23. | 23. |
| 24. | 24. |
| 25. | 25. |
| 26. | 26. |
| 27. | 27. |
| 28. | 28. |
| 29. | 29. |
| 30. | 30. |
| 31. | 31. |
| 32. | 32. |
| 33. | 33. |
| 34. | 34. |
| 35. | 35. |
| 36. | 36. |
| 37. | 37. |
| 38. | 38. |
| 39. | 39. |
| 40. | 40. |
| 41. | 41. |
| 42. | 42. |
| 43. | 43. |
| 44. | 44. |
| 45. | 45. |
| 46. | 46. |
| 47. | 47. |
| 48. | 48. |
| 49. | 49. |
| 50. | 50. |
| 51. | 51. |
| 52. | 52. |
| 53. | 53. |
| 54. | 54. |
| 55. | 55. |
| 56. | 56. |
| 57. | 57. |
| 58. | 58. |
| 59. | 59. |
| 60. | 60. |
Insulin Biosulin N suspension for subcutaneous injection 100 IU/ml 10 ml
Indications
- diabetes mellitus type 1;
- type 2 diabetes mellitus: stage of resistance to oral hypoglycemic agents, partial resistance to these drugs (during combination therapy), intercurrent diseases.
pharmachologic effect
Medium-acting insulin preparation. It is human insulin obtained using recombinant DNA technology.
Interacts with a specific receptor on the outer cytoplasmic membrane of cells and forms an insulin-receptor complex that stimulates intracellular processes, incl. synthesis of a number of key enzymes (including hexokinase, pyruvate kinase, glycogen synthetase). The decrease in blood glucose levels is due to an increase in its intracellular transport, increased absorption and assimilation by tissues, stimulation of lipogenesis, glycogenogenesis, and a decrease in the rate of glucose production by the liver.
The duration of action of insulin preparations is mainly determined by the rate of absorption, which depends on several factors (for example, on the dose, route and site of administration), and therefore the insulin action profile is subject to significant fluctuations, both between different and within the same patient .
After subcutaneous administration, the onset of action is observed after approximately 1-2 hours, the maximum effect is between 6 and 12 hours, the duration of action is 18-24 hours.
Drug interactions
There are a number of medications that affect the need for insulin.
The hypoglycemic effect of insulin is enhanced
oral hypoglycemic drugs, MAO inhibitors, ACE inhibitors, carbonic anhydrase inhibitors, non-selective beta-blockers, bromocriptine, octreotide, sulfonamides, anabolic steroids, tetracyclines, clofibrate, ketoconazole, mebendazole, pyridoxine, theophylline, cyclophosphamide, fenfluramine, lithium preparations, preparations containing ethanol .
The hypoglycemic effect of insulin is weakened
oral contraceptives, corticosteroids, thyroid hormones, thiazide diuretics, heparin, tricyclic antidepressants, sympathomimetics, danazol, clonidine, slow calcium channel blockers, diazoxide, morphine, phenytoin, nicotine, sulfinpyrazone, epinephrine, histamine H1 receptor blockers.
Under the influence of reserpine and salicylates, it is possible to both weaken and enhance the effect of the drug.
Dosage regimen
The dose of the drug is determined by the doctor individually, in each specific case, based on the concentration of glucose in the blood.
The drug is intended for subcutaneous administration. The average daily dose varies from 0.5 to 1 IU/kg body weight (depending on the individual characteristics of the patient and blood glucose levels).
The temperature of the administered insulin should be at room temperature.
Biosulin® N is injected subcutaneously into the thigh; injections can also be made into the anterior abdominal wall, buttock or deltoid muscle area.
It is necessary to change injection sites within the anatomical region to prevent the development of lipodystrophies.
Biosulin® N can be administered either as a single drug or in combination with short-acting insulin (Biosulin® R).
Injection technique when using insulin in vials
Do not use Biosulin® N if, when mixing the contents of the bottle according to the instructions for use, the insulin does not become uniformly white and cloudy. Do not use Biosulin® N if there are flakes in it after mixing. Do not use Biosulin® N if hard white particles have stuck to the bottom or walls of the bottle, creating a “frosty pattern” effect.
If the patient is using only one type of insulin
- The rubber membrane on the bottle should be disinfected.
- Draw air into the syringe in a volume corresponding to the required dose of insulin. Inject air into the insulin vial.
- Turn the bottle with the syringe upside down and draw the required dose of insulin into the syringe. Remove the needle from the vial and remove air from the syringe. Check that the insulin dose is set correctly.
- Inject immediately.
If a patient needs to mix two types of insulin
- The rubber membranes on the bottles should be disinfected.
- Immediately before drawing, roll the vial of long-acting insulin (“cloudy”) between your palms until the insulin becomes uniformly white and cloudy.
- Draw air into the syringe in a volume corresponding to the dose of “cloudy” insulin. Inject air into the bottle with “cloudy” insulin and remove the needle from the bottle (“cloudy” insulin should not be drawn at this stage yet).
- Draw air into the syringe in a volume corresponding to the dose of short-acting (“transparent”) insulin. Inject air into the vial with “transparent” insulin. Turn the bottle with the syringe upside down and draw the required dose of “transparent” insulin. Remove the needle and remove air from the syringe. Check that the dose taken is correct.
- Insert the needle into the bottle with “cloudy” insulin, turn the bottle with the syringe upside down and draw the required dose of insulin. Remove air from the syringe and check the correct dose. Immediately inject the collected insulin mixture.
- Insulins should always be taken in the same sequence described above.
Injection technique when using insulin cartridges
The cartridge with the drug Biosulin® N is intended for use with the BiomaticPen® or Biosulin® Pen syringe pen. The patient should be warned about the need to carefully follow the instructions in the instructions for using the syringe pen for administering insulin.
Before use, make sure that there is no damage (for example, cracks) on the cartridge with Biosulin® N. Do not use the cartridge if there is any visible damage. Do not use Biosulin® N if, when mixing the contents of the cartridge according to the instructions for use, the insulin does not become uniformly white and cloudy. Do not use Biosulin® N if there are flakes in it after mixing. Do not use Biosulin® N if hard white particles have stuck to the bottom or walls of the bottle, creating a “frosty pattern” effect.
After the cartridge is inserted into the pen, a colored stripe should be visible through the window of the cartridge holder.
Before placing the cartridge into the pen, you should turn the cartridge up and down so that the glass ball moves from end to end of the cartridge. This procedure should be repeated at least 10 times until all the liquid becomes white and uniformly cloudy. Immediately after this you need to give an injection.
If the cartridge is already inside the pen, you should turn it with the cartridge inside up and down at least 10 times. This procedure must be repeated before each injection.
After injection, the needle should remain under the skin for at least 6 seconds. You should keep the button pressed until the needle is completely removed from under the skin, thus ensuring the correct dose is administered and limiting the possibility of blood or lymph getting into the needle or into the insulin cartridge.
The cartridge with Biosulin® N is intended for individual use only and cannot be refilled.
Injection procedure
- Use two fingers to gather a fold of skin, then insert a needle into the base of the fold at an angle of about 45° and inject insulin under the skin.
- After injection, the needle must remain under the skin for at least 6 seconds to ensure that the insulin is completely injected.
- If blood appears at the injection site after removing the needle, lightly press the injection site with a swab moistened with a disinfectant solution (for example, alcohol).
- Injection sites should be changed.
Overdose
Symptoms:
hypoglycemia may develop.
Treatment:
The patient can eliminate mild hypoglycemia himself by ingesting sugar or carbohydrate-rich foods (diabetic patients are advised to carry sugar, sweets, cookies or sweet fruit juice with them at all times). In severe cases, in case of loss of consciousness, a 40% dextrose solution is administered intravenously; IM, SC or IV - glucagon. After regaining consciousness, the patient is advised to take a carbohydrate-rich meal to prevent the recurrence of hypoglycemia.
Contraindications for use
- hypoglycemia;
- hypersensitivity to insulin or other components of the drug.
Restrictions for children
No data
Use in elderly patients
The dose of the drug must be adjusted in persons over 65 years of age.
Restrictions for elderly patients
Use with caution
Use for liver dysfunction
The dose of the drug must be adjusted in case of liver dysfunction.
Restrictions for liver dysfunction
Use with caution
Use during pregnancy and breastfeeding
There are no restrictions on the treatment of diabetes mellitus with insulin during pregnancy, because insulin does not cross the placental barrier. When planning pregnancy and during it, it is necessary to intensify the treatment of diabetes mellitus. Insulin requirements usually decrease in the first trimester of pregnancy and gradually increase in the second and third trimesters.
During and immediately after childbirth, the need for insulin may decrease dramatically. Shortly after birth, insulin requirements return to pre-pregnancy levels.
There are no restrictions on the treatment of diabetes mellitus with insulin during breastfeeding. However, a reduction in the insulin dose may be necessary, so careful monitoring is necessary for several months until insulin requirements stabilize.
Restrictions when breastfeeding
Use with caution
Restrictions during pregnancy
Use with caution
Use for renal impairment
The dose of the drug must be adjusted if renal function is impaired.
Restrictions for impaired renal function
Use with caution
Storage conditions
The drug should be stored out of the reach of children, protected from light at a temperature of 2° to 8°C; do not freeze.
Terms of sale
The drug is available with a prescription.
special instructions
Do not use Biosulin® N if, after shaking, the suspension does not become white and uniformly cloudy.
During insulin therapy, constant monitoring of blood glucose levels is necessary.
The causes of hypoglycemia, in addition to insulin overdose, can be a change in drug, skipping meals, vomiting, diarrhea, increased physical activity, diseases that reduce the need for insulin (impaired liver and kidney function, hypofunction of the adrenal cortex, pituitary gland or thyroid gland), change of injection site, and also interaction with other drugs.
Incorrect dosing regimen or interruptions in insulin administration, especially in patients with type 1 diabetes mellitus, can lead to hyperglycemia. Typically, the first symptoms of hyperglycemia develop gradually, over several hours or days. They include the appearance of thirst, increased urination, nausea, vomiting, dizziness, redness and dryness of the skin, dry mouth, loss of appetite, and the smell of acetone in the exhaled air. If left untreated, hyperglycemia in type 1 diabetes can lead to life-threatening diabetic ketoacidosis.
The dose of insulin must be adjusted in case of dysfunction of the thyroid gland, Addison's disease, hypopituitarism, impaired liver and/or kidney function, diabetes mellitus in persons over 65 years of age.
Adjustment of the insulin dose may also be required if the patient increases the intensity of physical activity or changes the usual diet.
Concomitant diseases (especially infectious) and conditions accompanied by fever increase the need for insulin.
The transition from one type of insulin to another should be carried out under the control of blood glucose levels.
The drug reduces tolerance to alcohol.
Due to the possibility of precipitation in some catheters, the use of the drug in insulin pumps is not recommended.
Impact on the ability to drive vehicles and machinery
With the initial prescription of insulin, changing its type, or with significant physical or mental stress on the body, the ability to drive a car or operate various mechanisms, as well as engage in other potentially hazardous activities that require increased attention and speed of psychomotor reactions, may be reduced.
Side effect
Caused by the effect on carbohydrate metabolism:
hypoglycemic conditions (pallor of the skin, increased sweating, palpitations, tremor, hunger, agitation, paresthesia in the mouth, headache). Severe hypoglycemia can lead to the development of hypoglycemic coma.
Allergic reactions:
skin rash, angioedema, anaphylactic shock.
Local reactions:
hyperemia, swelling and itching at the injection site; with long-term use - lipodystrophy at the injection site.
Other:
swelling, transient refractive errors (usually at the beginning of therapy).
Possible product names
- Insulin Biosulin N susp s.c. 100IU/ml 10ml
- BIOSULIN N 100 UNITS/ML 10 ML
- BIOSULIN H SUSP. P/C ENTER. 100 IU/ML FL. 10 ML. X1
- BIOSULIN N SUSPENSION FOR SC INJECTION. 100IU/ML FL. 10ML №1(INSULIN)
- BIOSULIN N 100 IU/ML SUSP. FOR P/LEATHER. ENTER 10ML FL. X1 B (R)
- (Biosulin N) Insulin Biosulin N suspension subcutaneous 100 IU/ml 10 ml